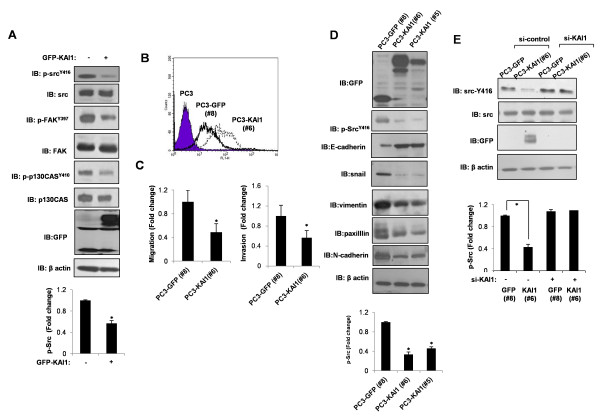
Figure 1.
Phosphorylation of Src at Tyr416 is inhibited by KAI1. (A) After transient transfection of KAI1 in PC3 prostate cancer cells, phospho-SrcY416, phospho-FAKY397, and phospho-p130CasY410 protein levels were measured by immunoblotting. Densitometric analysis of phospho-Src Y416 was shown after normalization to beta-actin. (B) Vector-transfected cells (PC3-GFP clone #8) and KAI1-expressing PC3 clones (PC3-KAI1 clone #5 and #6) were picked after stable transfection with KAI1 cDNA and selection in 800 μg/ml G418. Cell surface expression of KAI1 was analyzed by flow cytometry. (C) The inhibitory effect of KAI1 on migration and invasion was confirmed in KAI1-expressing clones by transwell migration assay and invasion assay, respectively. (D) Cell lysates were harvested and immunoblotted for GFP-KAI1, phospho-SrcY416, E-cadherin, Snail, vimentin, paxillin, and N-cadherin. Densitometric analysis of phospho-Src Y416 was shown after normalization to beta-actin. (E) Vector-transfected cells (PC3-GFP #8), and KAI1-expressing PC3 clones (PC3-KAI1 #6) were transiently transfected with siRNA against KAI1, and then Src phosphorylation was monitored by immunoblotting. Densitometric analysis of phospho-Src Y416 was shown after normalization to beta-actin.