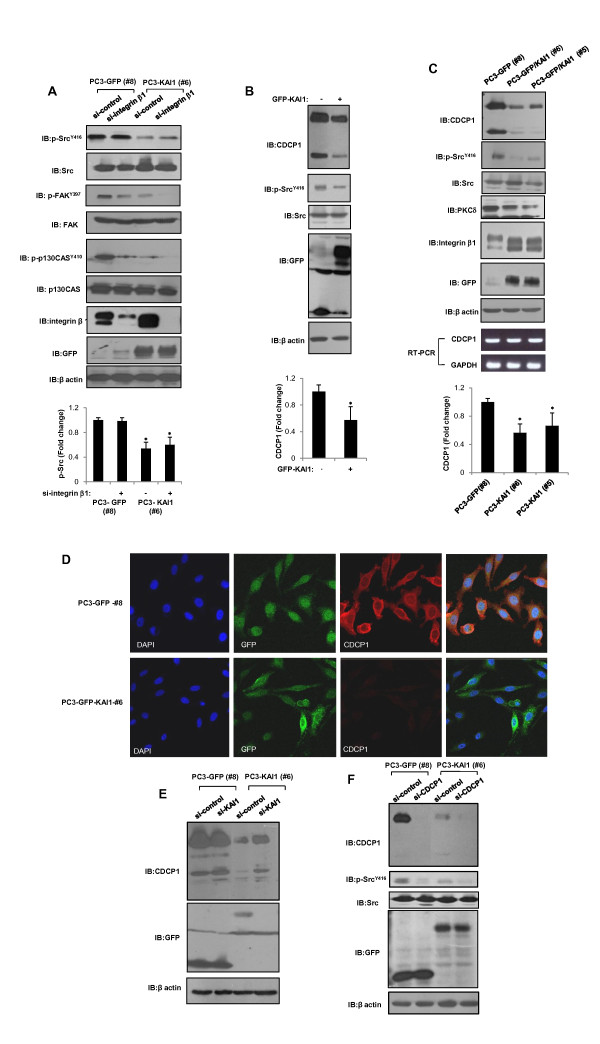
Figure 2.
KAI1-mediated negative regulation of CDCP1 inhibits Src. (A) Vector-transfected cells (PC3-GFP #8) and KAI1-expressing PC3 clones (PC3-KAI1 #6) were transiently transfected with siRNA against integrin β1. Forty-eight hours after transfection, the levels of phospho-SrcY416, phospho-FAKY397, phospho-p130CasY410, and integrin β1 were measured by immunoblotting. Densitometric analysis of phospho-Src Y416 was shown after normalization to beta-actin. (B) PC3 cells were transfected with KAI1. Twenty-four hours after transfection, phospho-SrcY416 and CDCP1 were detected by immunoblotting. Densitometric analysis of CDCP1 was shown after normalization to beta-actin. (C) Cell lysates from PC3 vector control (PC3-GFP #8) and KAI1 transfectants (PC3-KAI1 #5 and PC3-KAI1 #6) were analyzed for CDCP1, phospho-SrcY416, PKCδ, integrin β1, and GFP-KAI1 protein levels. Densitometric analysis of CDCP1 was shown after normalization to beta-actin. (D) CDCP1 proteins were detected by immunofluorescence using anti-CDCP1 antibody and visualized using rhodamine-labeled secondary antibody. (E) PC3 cells stably transfected with KAI1 (PC3-KAI1 #6) and vector control (PC3-GFP #8) cells were treated with siRNA against KAI1. After 48 hours, cell lysates were analyzed by immunoblotting to detect CDCP1 and GFP-KAI1. (F) After siRNA-mediated knockdown of CDCP1, phospho-SrcY416 levels were monitored by immunoblotting.