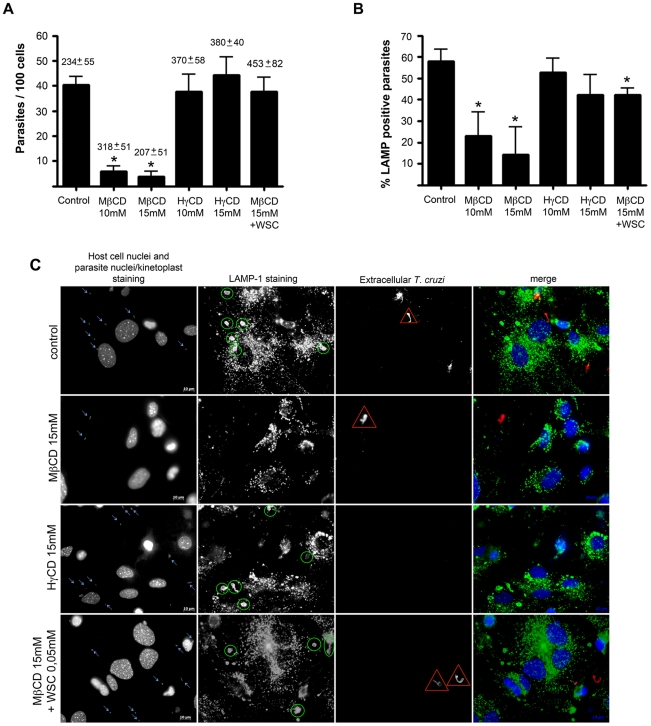
Figure 2. T. cruzi invasion of cells and association with LAMP-1 in cardiomyocytes decreases after cholesterol depletion.
Cardiomyocytes pre-treated or not with different cyclodextrins were washed and challenged with T. cruzi trypomastigotes at a M.O.I of 50, for 40 minutes at 37°C, then fixed and processed for immunofluorescence detection of total intracellular parasites, as well as intracellular parasites associated with LAMP-1 (a lysosomal marker). Both T. cruzi internalization (A) and association with host LAMP-1 (B) diminishes after incubation with 10 and 15 mM of MβCD but not after treatment with 10 and 15 mM of HγCD. Cholesterol replenishment after treatment with 15 mM MβCD reverts the effect of the drug on parasite cell invasion (A), and at least partially on LAMP-1 association (B). The average number of cardiomyocytes ±SD per 10 counted fields in each coverslip is shown above the bars (A). Data are shown as mean of triplicates ±SD. Asteriks indicate statistically significant differences (p < 0.05, Student's t test) between control and treated cells. (C) Representative panels of T. cruzi invasion and association with host cell lysosomes, revealed by immunocytochemistry. Total cell and parasite nuclei, as well as parasite kinteoplast DNA were labeled with DAPI; lysosomes were labeled with anti-LAMP 1 antibody followed by secondary IgG labeled with Alexa Fluor 488; extracellular parasites in the field were labeled with anti-T.cruzi antibody followed by secondary IgG labeled with Alexa Fluor 546. From top to bottom: control cells, 15 mM MβCD treated cells, 15 mM HγCD treated cells and 15 mM MβCD treated cells followed by incubation with 0.05 mM of WSC. Blue arrows show total T. cruzi trypomastigotes in the field, yellow ellipsoids show lysosomal associated trypomastigotes, red triangles points out extracellular trypomastigotes and the last column shows the merge of the three previous. Scale bar: 10 µm.