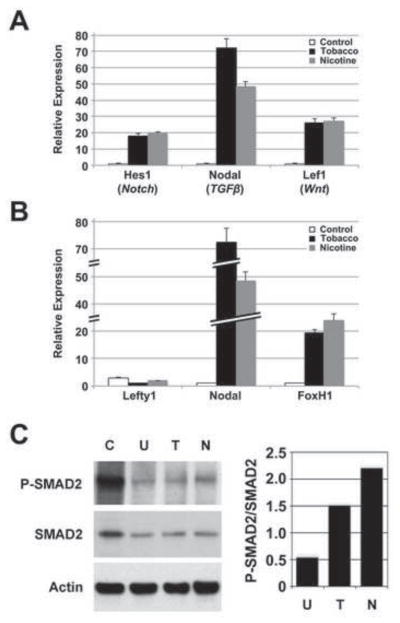
Fig. 5. Nodal signaling mediates the effects of tobacco smoke on differentiation.
hESCs were cultured in differentiation conditions for 14 days in control, nicotine-supplemented, or tobacco smoke-exposed media. A) Expression of genes associated with Notch (Hes1), TGFβ (Nodal) and canonical Wnt (Lef1) signaling pathways was evaluated by quantitative real-time PCR. Data shown are mean±SD (N=3). While all three developmentally important pathways were affected by tobacco smoke exposure, the greatest effect was seen with Nodal. In addition, the significant difference between Nodal upregulation with tobacco smoke and nicotine alone suggested that the effects of tobacco smoke on Nodal occur through nicotine-dependent and -independent mechanisms. B) Expression of Nodal, the Nodal inhibitor, Lefty1, and the Nodal effector, FoxH1, was evaluated by quantitative real-time PCR. Data shown are mean±SD (N=3). While the Nodal effector, FoxH1, was upregulated with tobacco smoke and nicotine exposure, Lefty1, a Nodal inhibitor, was downregulated, implicated the Nodal pathway in mediating tobacco smoke related effects. C) Whole cell lysate from Activin A-stimulated, undifferentiated hESCs (C; positive control), and hESCs differentiated for 14 days and treated with tobacco smoke- (T) or nicotine-supplemented medium (N), or untreated medium (U), were analyzed by immunoblot for total SMAD2 and phosphorylated SMAD2 (P-SMAD2) expression (left panel). Actin expression was used as a loading control. Immunoblot signals for SMAD2 and P-SMAD2 were quantified, normalized to Actin signals, and expressed as a ratio of P-SMAD2:SMAD2 (right panel). Typical results are shown. There was no difference in SMAD2 expression between samples, however, P-SMAD2 was upregulated with tobacco smoke exposure and more so with nicotine treatment, consistent with a delay in differentiation. The increase in SMAD2 phosphorylation with nicotine compared to tobacco smoke exposure suggests that nicotine may be the predominant effector of Nodal signaling through SMAD2, even though Nodal expression was higher with total tobacco smoke.