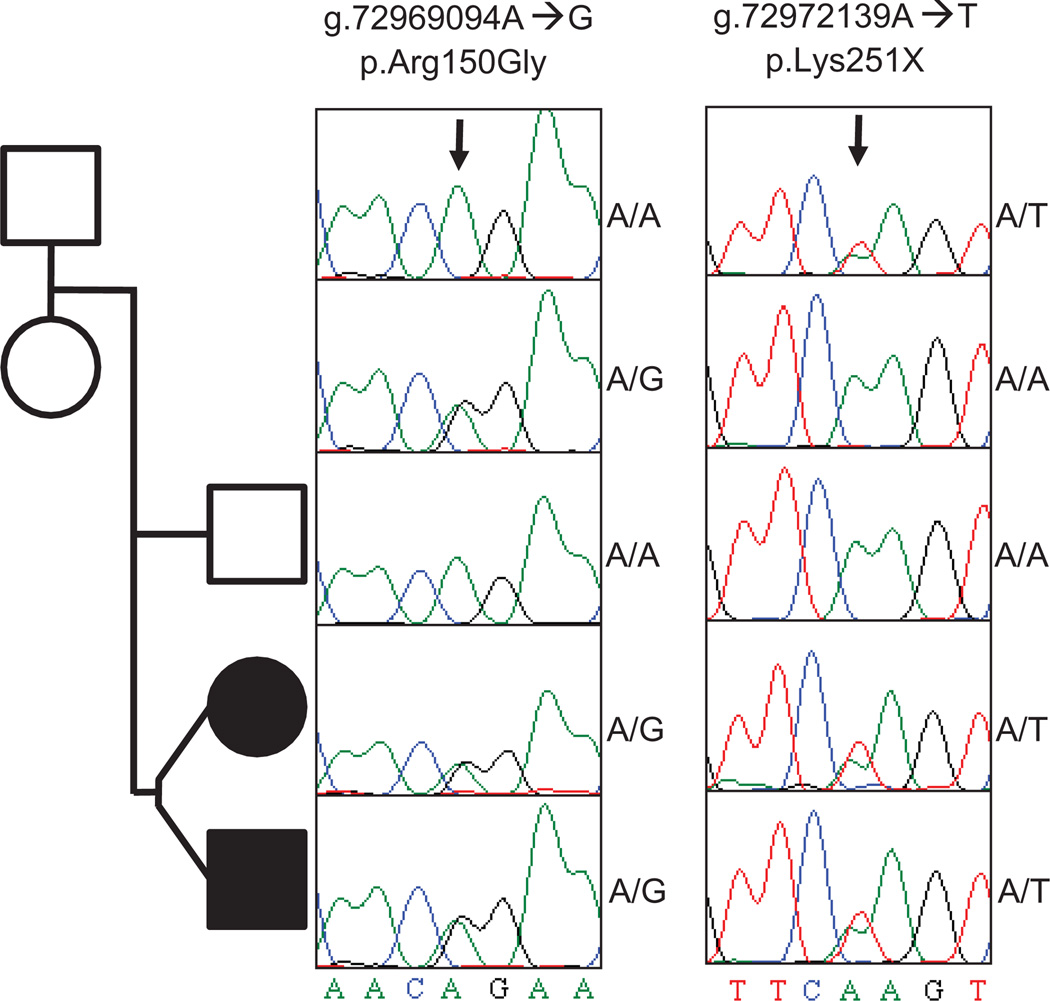
Fig. 3.
Validation and segregation of two deleterious SPR alleles. Shown is the pedigree of the two DRD-affected probands (shaded), their unaffected sibling, and their parents. Squares indicate male subjects and circles female subjects. The Sanger sequencing traces showing the SPR genotype of each member of the pedigree are shown. p.Arg150Gly refers to the A→G mutation on chromosome 2 at nucleotide 72,969,094, leading to the amino acid missense mutation; p.Lys251X indicates the A→T mutation on chromosome 2 at nucleotide 72,972,139, causing an amino acid nonsense mutation. The variants are deleterious to SPR activity but have only been observed, separately, as homozygous mutations from presumed consanguineous kindreds. The unaffected mother is homozygous (A/A) for the wild-type allele at the first locus but heterozygous (A/T) for the pathogenic p.Lys251X allele at the second locus. Similarly, the unaffected father is heterozygous (A/G) for the pathogenic p.Arg150Gly allele at the first locus and homozygous (A/A) for the wild-type allele at the second locus. Each DRD-affected proband is heterozygous (A/G and A/T) for the pathogenic mutations at both alleles, indicating a new compound heterozygous combination of mutant alleles that is causative for DRD. The mutations result in reduction of SPR-mediated synthesis of BH4, a coenzyme needed for neurotransmitter production. Absence or reduction of the neurotransmitter dopamine causes distinct clinical symptoms including dystonia.