Abstract
Sarcoidosis is a systemic, clinically heterogeneous disease characterized by the development of granulomas. Any organ system can be involved, and patients may present with any number of rheumatologic symptoms. There are no U.S. Food and Drug Administration–approved therapies for the treatment of sarcoidosis. Diagnosing sarcoidosis becomes challenging, particularly when its complications cause patients’ symptoms to mimic other conditions, including polymyositis, Sjögren syndrome, or vasculitis. This review presents an overview of the etiology of and biomarkers associated with sarcoidosis. We then provide a detailed description of the rheumatologic manifestations of sarcoidosis and present a treatment algorithm based on current clinical evidence for patients with sarcoid arthritis. The discussion will focus on characteristic findings in patients with sarcoid arthritis, osseous involvement in sarcoidosis, and sarcoid myopathy. Arthritic conditions that sometimes coexist with sarcoidosis are described as well. We present two cases of sarcoidosis with rheumatologic manifestations. Our intent is to encourage a multidisciplinary, translational approach to meet the challenges and difficulties in understanding and treating sarcoidosis.
Keywords: Sarcoidosis, rheumatic diseases, arthritis, myopathy
Sarcoidosis is a multisystem disease of unknown etiology. Patients with sarcoidosis may develop extrapulmonary organ involvement, including rheumatologic complications. Here we will briefly review the etiology of sarcoidosis and then describe the presentations of and diagnostic findings in various rheumatologic manifestations of sarcoidosis. To highlight some of the rheumatologic complications of sarcoidosis, we also present one case of peripheral sarcoid arthritis and one case with axial sarcoid involvement. We then propose an approach for the management of patients with sarcoid arthritis in particular, an approach that borrows on the effective strategies employed for rheumatoid arthritis.
ETIOLOGY
Despite significant advances in characterizing features of sarcoidosis, the underlying etiology remains to be elucidated. Genetic, environmental, and infectious links have all been suggested with varying levels of supportive evidence. Strong evidence suggests that a genetic component confers increased susceptibility to the disease. Previous genetic studies in sarcoidosis have established a role for variants in the class I and II human leukocyte antigen (HLA) locus, similar to many other autoimmune diseases. Recently, genomewide association studies (GWASs) have been performed in sarcoidosis to more comprehensively detect genes associated with sarcoidosis susceptibility. A recent report from these ongoing studies identified annexin A11 (ANXA11) as a novel non-HLA susceptibility locus for sarcoidosis in 2008.1 Associations have been found between risk of sarcoidosis and as well as genetic polymorphisms involving the loci coding for tumor necrosis factor-alpha (TNF-α), co-stimulatory molecules on antigen-presenting cells such as CD80 and CD86, chemokine receptors CCR2 and CCR5, and many others.2,3 These studies have yet to explain much of the heritability of sarcoidosis, and increasing insights into the genetics of sarcoidosis susceptibility and severity are anticipated.
It is unclear whether the Th1 immune response seen in sarcoidosis is directed at one or more specific antigens or is a function of a generalized immune dysfunction, because studies have provided evidence for both propositions. Environmental exposure seems to play a role as well. Modest positive associations have been demonstrated in patients exposed to agricultural settings, mold, mildew, musty odors, and pesticides.2 Patients isolated from such exposures (e.g., office workers) have a reduced risk of developing sarcoidosis.2
Seasonal and geographic clustering of new cases of sarcoidosis,4 in addition to the apparent transmissibility between organ donors and recipients,4 support the long-standing notion that an infectious agent may be involved. Numerous studies have demonstrated the presence of mycobacterial antigens within biopsies of sarcoid granulomas, though the association is not consistent because only 26% of samples bear the remnants of the organism.2,4 DNA and protein fragments from Propionibacterium acnes have been found in sarcoidosis tissues as well, with some evidence suggesting a maladaptive response to the bacterial antigens in affected individuals.5 The ubiquitous presence of Propionibacteria in even healthy individuals necessitates an understanding of how pathological conversion occurs in sarcoidosis, a topic that warrants further study.2,4,5 A number of other infectious agents such as Borrelia burgdorferi have also shown positive associations, though the studies have been limited in size and the evidence is not as strong at this time.5
RHEUMATOLOGIC MANIFESTATIONS OF SARCOIDOSIS
Sarcoidosis and Lupus Erythematosis
Systemic lupus erythematosis (SLE) may develop in the context of sarcoidosis. SLE is usually suspected when patients with sarcoidosis develop a butterfly rash or discoid lesions. The characteristic appearance of a butterfly rash is a large, erythematous patch usually confined to the nose and cheeks.6 SLE discoid lesions are scaly, circular lesions with depigmented centers surrounded by an erythematous perimeter. Sarcoid and discoid lupus skin changes may coexist in the same lesion (Fig. 1). The treatment options for sarcoidosis and SLE are challenging and should be individualized. The use of anti-TNF-α agents may be associated with drug-induced lupus and should be avoided in patients who have active systemic lupus.
Figure 1.
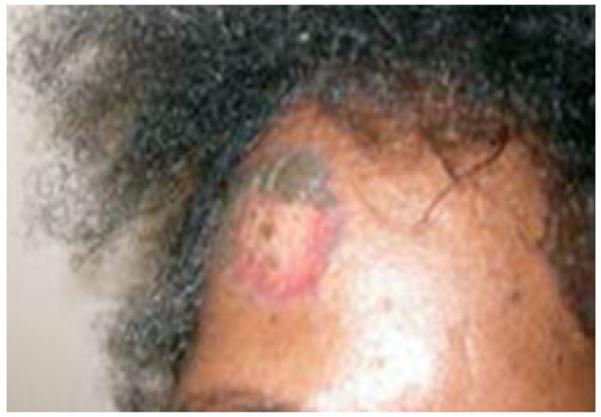
photograph of the frontal area showing erythematous rash. Biopsy confirmed features of discoid lupus and noncaseating granulomas in the same lesion.
Osseous Sarcoidosis
Osseous lesions are found in up to 13% of patients with sarcoidosis.7 Because osseous sarcoidosis is often asymptomatic, this estimation may not reflect the true prevalence.8 The presence of sarcoidosis in the bones typically correlates with cutaneous lesions and progressive disease.9,10 In osseous sarcoidosis, bilateral involvement of the phalanges of the hand and feet is most common,11 but any bone may be affected. Reports of leg (e.g., tibial), skull, rib, sternal, and vertebral involvement are rare but do exist.7,8,11,12 When present, symptoms include focal pain, swelling and erythema in the subcutaneous tissue around the involved bone site.10 Osseous sarcoidosis is detected by magnetic resonance imaging (MRI), bone scans, or plain film imaging. A specific diagnostic algorithm has yet to be defined.12
AXIAL SARCOIDOSIS
Involvement of the axial skeleton as a specific form of osseous sarcoid is rare and can be misdiagnosed as sacroiliitis by plain radiograph. Sometimes, vertebral lesions are asymptomatic and never require treatment. In other cases, vertebral sarcoidosis may be the presenting symptom; when it is symptomatic, it is often quite painful.13 Lesions may be predominantly lytic, sclerotic, or a mixture of the two, as determined by radiographic images of the lower dorsal, upper lumbar, and cervical vertebrae.14 MRI has been suggested as the preferred imaging modality because it can differentiate sacroiliitis from osseous lesions of sarcoidosis, and it can guide selection of biopsy sites to confirm the diagnosis histo-pathologically if that is indicated.14 MRI findings include multifocal lesions that are hypointense on T1-weighted imaging and hyperintense on T2-weighted imaging.13,15 MRI findings in osseous sarcoid are not specific, and metastatic cancer, myeloma, lymphoma, and tuberculosis must be considered,16 making biopsy an important diagnostic step. Optimal treatment of axial sarcoidosis remains controversial. There are no controlled clinical trials, but corticosteroid-based regimens are the standard of care. Other treatment options, including TNF-α inhibitors (infliximab and adalimumab), have been described in cases of refractory to standard treatment.17
SACROILIAC JOINT INVOLVEMENT
Sacroiliitis, arthritis of the sacroiliac joints, may cause lower back pain and stiffness, with pain extending from the lower back to the buttocks. Although it is rare, sacroiliitis may occur in patients with sarcoidosis. Evidence for sacroiliitis as a manifestation of sarcoidosis comes mostly from isolated cases.18 The frequency of sacroiliitis in sarcoidosis was 6.6% in one study.19 Non-caseating granulomas have been seen on biopsy of the sacroiliac joint, which supports an etiologic role of sarcoidosis in sacroiliitis in patients with both conditions.20 However, sacroiliitis may present in the context of other rheumatologic conditions, such as reactive arthritis, ankylosing spondylitis, and psoriatic spondyloarthropathy, which may coexist with sarcoidosis, and these conditions should therefore be ruled out patients with sarcoid and sacroiliitis findings.19 Typically, the back pain of sacroiliitis can be managed with physiotherapy and analgesia. However, in the context of sarcoidosis, inflammatory back disease may be more effectively treated with targeted therapies, including antiinflammatory medication and local steroid injections.19
Sarcoid Myopathy
Sarcoid myopathy occurs more frequently than osseous sarcoid, affecting up to 75% of all individuals with sarcoidosis. Similar to bone involvement, myopathy is associated with chronic disease. Muscle involvement is often asymptomatic. In suspected cases, muscle biopsy reveals noncaseating granulomas (Fig. 2). Helpful imaging techniques include MRI, computed tomographic (CT) scan, or positron emission tomography (PET) where myopathy appears as star-shaped lesions within the muscle.9,21,22 Sarcoid myopathy may manifest as nodular myopathy, chronic myopathy, or acute myositis.23 Nodular myopathy is rare24 and manifests as multiple, tumorlike, palpable nodules in the muscles.25 Chronic myopathy occurs when myopathy is present in multiple muscle groups. Corticosteroids have been used to treat chronic myopathy, but patients—who tend to be severely disabled—rarely improve.25 Corticosteroids, azathioprine, and methotrexate are used for severe, symptomatic nodular and chronic sarcoid myopathy, but optimal treatment strategies have not been fully established,24 and patients who are already significantly disabled rarely improve.
Figure 2.
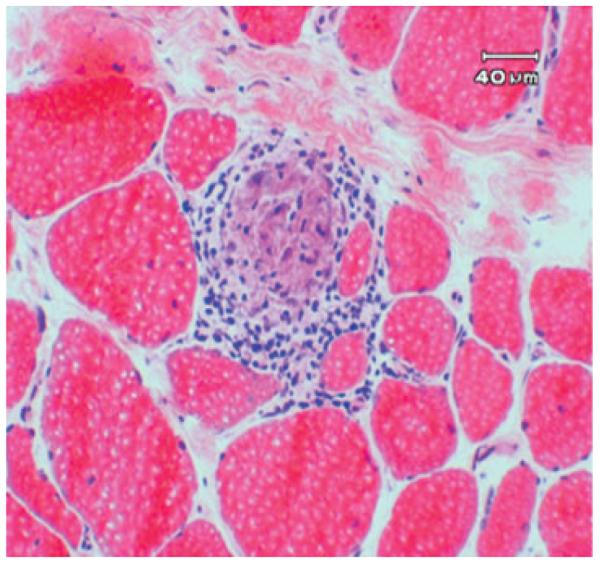
resence of noncaseating granulomas in muscle in sarcoid myopathy.
The least common type of sarcoid myopathy is acute myositis. It usually occurs in the setting of acute arthritis but due to a low prevalence its normal characteristics, prognosis, and preferred treatment approaches are not well defined.22,25,26 It tends to occur early in the course of sarcoidosis and in patients younger than 40 years old.25,27 Patients with acute myositis typically have muscle swelling and experience diffuse pain emanating from the calf or thigh. Sometimes, sarcoid myositis may lead to contracture of the muscle and hypertrophy.28 Nonspecific associated symptoms include fatigue and fever.27 Generalized muscle weakness occurs infrequently.27 Because the clinical presentation of patients with acute myopathy can mimic polymyositis (i.e., elevated muscle enzymes and abnormal findings on electromyogram), muscle biopsy may be needed to distinguish between the two conditions. Methotrexate has shown efficacy in the treatment of acute sarcoid myositis.23 The role of intravenous immunoglobulins, mycophenolate, and anti-TNF-α therapy in muscle sarcoidosis is not well defined.
Although there is no apparent pathophysiological link, an association between sarcoid and inclusion body myositis was reported in 1986.29 However, very few cases—eight as of 2008—have been reported.30,30a Of note, the immunopathology of both diseases involves Th1-mediated immunity, and some evidence suggests that muscle involvement in sarcoid may lead to the development of inclusion body myositis,30 although a detailed understanding of the relationship between these two diseases is not yet fully known.
Sarcoidosis and Vasculitis
Systemic vasculitis associated with sarcoidosis is uncommon but can cause significant morbidity when it occurs. Vasculitis in patients with sarcoidosis can affect vessels that range in size from small to large and may manifest as cutaneous lesions, neuropathy, pulmonary hypertension, or systemic vasculitis.23 Other symptoms that have been reported in a few cases of sarcoid vasculitis include Takayasu-like aortitis, vasculitis of the aortic branches, glomerulonephritis, and transient cerebral ischemia.31 In our experience a subset of patients with sarcoidosis may have positive antineutrophilic cytoplasmic antibodies (ANCAs) in the absence of systemic vasculitis (unpublished data). However, the role of these antibodies in the pathogenesis of sarcoid vasculitis remains unknown.
Treatment of sarcoid vasculitis with corticosteroids may be successful initially, but relapse is common. Anecdotal reports suggest that corticosteroids may be helpful but the length of therapy is unknown. Similarly, the role of steroid-sparking agents remains undefined. According to new data from the Rituximab for ANCA-Associated Vasculitis (RAVE) trial, rituximab is non-inferior to cyclophosphamide in treating vasculitis.32 The role of rituximab in sarcoidosis is not yet defined but we are currently conducting a pilot study of rituximab in pulmonary sarcoidosis.
Sjögren Syndrome and Sarcoidosis
Occasionally sarcoidosis and Sjögren syndrome, both chronic inflammatory conditions that can affect the salivary glands, occur simultaneously, although the incidence of coexistence is unknown.33 Dryness and diffuse swelling of oral mucosal tissues can be the first symptom of sarcoidosis.34 Both sarcoidosis and Sjögren syndrome have an insidious onset, and patients who do not present with other manifestations of sarcoidosis may appear to have isolated Sjögren syndrome.35 However, a detailed medical history, salivary gland biopsy, and serologies can help distinguish between the two conditions. Certain systemic complications such as uveitis, when present, can be more supportive of a diagnosis of sarcoid. Also, while patients with either sarcoidosis or Sjögren syndrome may demonstrate reduced stimulated secretory function of the parotid glands, sarcoid patients will not have elevated salivary sodium concentrations and globular sialectasia, which are features unique to Sjögren syndrome.36 Finally, elevated levels of SSA and SSB antibodies are usually positive in Sjögren syndrome and are not seen in sarcoidosis. Making a diagnosis of Sjögren syndrome currently requires sarcoidosis to be ruled out first. However, with accumulating evidence that Sjögren syndrome and sarcoidosis can coexist these criteria may change, and clinicians may need to evaluate for both conditions to determine whether a patient suffers from a single or a combined disorder.23,35 Objective evaluation of keratoconjunctivitis sicca by ophthalmologic exam is helpful as a baseline measurement. Lip biopsy may also be useful in making a Sjögren syndrome diagnosis. However, not all patients who have clear criteria for Sjögren syndrome require a lip biopsy, particularly individuals taking immunosuppressives in whom a lip biopsy might appear normal. A retrospective study found that the presence of sicca symptoms and positive serology accurately predict the outcomes of lip biopsies, suggesting that there is not a large role for lip biopsy in making an accurate diagnosis.37
Studies show that a cyclosporine ophthalmic emulsion (Restasis, Allergan, Inc., Irvine, CA) may be helpful to control sicca.38 However, the exact role of cyclosporine ophthalmic emulsion, and other agents, including pilocarpine hydrochloride (Salagen, Eisai, Inc., Woodcliff Lake, NJ) and cevimeline hydrochloride (Evoxac, Daiichi Sankyo, Inc., Parsippany, NJ), in the treatment of sicca associated with sarcoidosis is not well defined.
Articular Involvement
Up to 25% of patients with sarcoidosis have joint involvement.39–42 Japanese people with sarcoidosis are a notable exception, with very few cases of sarcoid arthritis reported and only 1.6% of patients with arthralgia.43,44 Traditionally, sarcoidosis arthritis is classified into two types: an acute, transient type, or a persistent, chronic type. The latter type is rare, occurring in only 1 to 4% of patients.45
ACUTE SARCOID ARTHRITIS
Acute sarcoid arthritis often occurs in the context of Löfgren syndrome, which is defined as the triad of erythema nodosum, bilateral hilar lymphadenopathy, and the presence of arthritis or arthralgias. A seasonal association between the initial presentation of acute sarcoid arthritis has been reported in some studies. Evidence suggests that initial presentations are clustered in the springtime, a notable difference from other arthritides, which tend to occur equally throughout the year.42,46,47
Visser and colleagues proposed a set of criteria for clinicians to use as a guide to help diagnose sarcoid arthritis. According to these criteria, making a diagnosis of articular sarcoidosis in patients who have three of the following four characteristics is 99% sensitive and 93% specific: (1) erythema nodosum, (2) symptom duration less than 2 months, (3) age less than 40, and (4) symmetrical ankle arthritis.42 Involvement of the ankle in acute sarcoid arthritis has been reported in 77 to 100% of patients, and symmetrical involvement occurs in most of those cases.41,42,46,47,49 Following the ankle, the next most common sites of pain and inflammation are the knee, wrist, and metacarpophalangeal joints. Patients frequently have an increased erythrocyte sedimentation rate,42,47 but other associated findings in acute sarcoid arthritis may vary among patients. For example, erythema nodosum may or may not be present. In the literature, the incidence is wide ranging (25 to 87.8%).42,44,46–49 Similarly, fever is noted in only one third of patients in some reports but in up to two thirds of patients in other case series.41,42,46,47
In the vast majority of cases, acute sarcoid arthritis has a benign, self-limited course, with minimal to no joint destruction. The average duration of symptoms is approximately 2 to 3 months, and most patients go into remission by 6 months after receiving nonsteroidal anti-inflammatory drugs or steroids.39,41,46–51
CHRONIC SARCOID ARTHRITIS
Chronic sarcoid arthritis typically occurs in the setting of systemic sarcoidosis. Chronic sarcoid arthritis typically involves the knees, ankles, wrists, hands, and/or feet. Joint destruction or Jaccoud deformity, when it occurs, is due to persistent inflammation.40,41 Joint effusions, synovitis, or even nodular proliferation of the synovium presenting as an intraarticular knee mass may also be present.45 The differential diagnosis of chronic sarcoid arthritis includes reactive arthritis and rheumatoid arthritis, which should be considered, particularly if there is symmetric disease and an elevated rheumatoid factor. Synovial fluid analysis typically reveals a milder inflammatory infiltrate in sarcoid arthritis than rheumatoid arthritis or infectious arthritis, although sometimes a synovial biopsy may be needed to definitely distinguish rheumatoid arthritis from sarcoid arthritis.40
MONOARTHRITIS, OLIGOARTHRITIS, POLYARTHRITIS
The majority of cases of sarcoid arthritis, whether acute or chronic, are oligoarthritic or polyarthritic (i.e., involving three or more joints). When patients with sarcoidosis present with symmetric polyarthritis, especially of the small joints of the hands, clinicians should evaluate patients for concomitant rheumatoid arthritis. Differentiating between the various types of arthritis should be based on clinical laboratory evaluations and radiographic imaging. Psoriatic arthritis, reactive arthritis, and gouty arthritis should also be considered in patients with sarcoidosis who present with oligoarthritis or monoarthritis. In immunocompromised patients, infectious arthritis needs to be excluded.
Monoarthritis is rare, although the incidence is variable in literature reports, with one prospective study citing only one of 28 patients having single joint involvement and another study reporting 21% of patients with Löfgren syndrome with single joint involvement.41,49 Similar to a polyarthritis presentation, when patients present with monoarthritis they should be evaluated for gouty arthritis and septic arthritis as well as CPP (calcium pyrophosphate)-positive arthritis.
PERIARTHRITIS
Patients with sarcoid arthritis often have periarticular inflammation.9,52 Despite swelling around the joints, individuals with periarthritis generally maintain normal range of motion.52
At times it may be difficult to distinguish between arthritis or periarthritis. Kellner and colleagues performed ultrasonography on 24 patients who presented with sarcoidosis involving the joints. Twenty of the 24 patients had subcutaneous or periarticular inflammation. Although eight patients had tenosynovitis, only six had discernible joint effusions.53 When diagnosing peri-arthritis, it is important to exclude lymphangitis, gonococcal infection, and human immunodeficiency virus (HIV) infection, especially in those who may be at risk for developing these infections.
COSTOCHONDRAL INVOLVEMENT
Costochondral rib junctions and chondrosternal joints of the chest wall occasionally become inflamed in patients with sarcoidosis. This complication, sometimes referred to as Tietze syndrome, creates intense pain that can radiate throughout the chest cavity. Usually, only the second or third rib and associated cartilage is affected. Analgesics are commonly used for pain control, although corticosteroids may be helpful in severe cases. Other than sarcoidosis, rheumatologic diseases, and infectious and neoplastic disorders should be considered in the differential diagnosis for costochondral symptoms.54 In our own clinical experience, we have injected corticosteroids directly into the costochondral joint in some patients in order to avoid systemic effects of oral corticosteroids.
GOUT IN SARCOIDOSIS
A relationship between gout and sarcoidosis has been appreciated for decades, although these observations were initially controversial. The first reported case of sarcoidosis, by Jonathan Hutchinson more than 120 years ago, was found to have coexistent sarcoidosis and gout.55 Interestingly, renal failure from gout was said to be the cause of eventual death of this patient. However, it has been suggested that sarcoidosis-associated hypercalcemia, hypercalciuria, or kidney stones may have been the true culprit.55 Later, Löfgren noted the association between sarcoidosis and altered urate metabolism, specifically leading to hyperuricemia. Kaplan and Klatskin then reported three cases of presumed simultaneous sarcoidosis, psoriasis, and gout. Despite the fact that the authors later concluded that these patients actually suffered from sarcoid arthritis that mimicked gout, they established a relationship between gout and sarcoidosis, of which clinicians are now aware.56,57 Differentiating between gout and sarcoid arthritis remains difficult since hyperuricemia can be present in both.58 Because both conditions are associated with increased morbidity, and because treatments differ considerably, correctly distinguishing between gout and sarcoid arthritis is important. The diagnosis of gouty arthritis should be confirmed by joint aspiration and documentation of the presence of sodium monourate crystals because the presence of hyperuricemia alone is not sufficient for a diagnosis of gouty arthritis.
SARCOIDOSIS AND PSORIATIC ARTHRITIS
Approximately 6% of all patients with sarcoidosis develop a psoriatic arthritis form of arthritis.42 The hall-mark feature of psoriatic arthritis is dactylitis, characterized by sausage-shaped digits.59,60 The patho-physiology in psoriatic arthritis in sarcoid may be different from that of nonsarcoid dactylitis. The management of psoriatic arthritis in sarcoidosis includes use of immunosuppressive, antiinflammatory, and biologic therapies.59,60 Of these agents, accumulating evidence supports that patients with psoriatic arthritis in the context of sarcoidosis may benefit from treatment with infliximab in particular.59 Although anti-TNF-α therapy is helpful in psoriasis,61 it may also paradoxically induce progressive psoriasis, and patients on these medications should be followed carefully.62
APPROACH FOR MANAGEMENT OF SARCOID ARTHRITIS
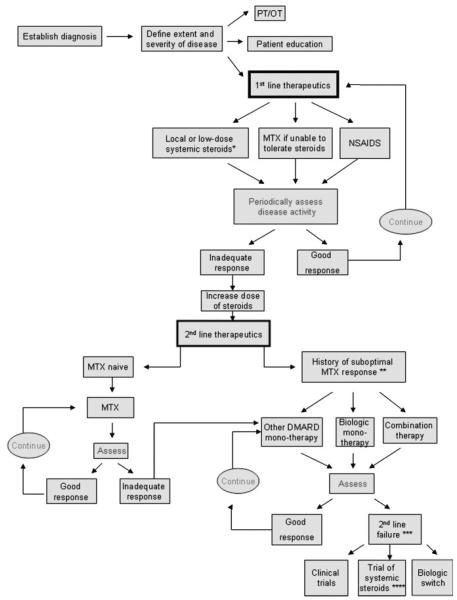
There are no U.S. Food and Drug Administration (FDA)-approved therapies for sarcoidosis in general, or for any of its manifestations in particular. Our proposed approach for the management of patients with sarcoid arthritis is shown in Figure 3. After establishing a diagnosis, first-line therapies include nonsteroidal antiinflammatory drugs (NSAIDs), local or low-dose systemic steroids, or methotrexate. Other agents may be considered depending on the severity of the signs and symptoms as well as the radiographic progression. Periodic disease assessments are required to determine whether the response is adequate. If the response is adequate, we continue treating with the first-line agents that are working well. Nonresponders may be tried on a higher dose of systemic steroids, or advanced directly to second-line treatments. Second-line therapies include methotrexate if patients are methotrexate-naïve or, in patients previously tried unsuccessfully on methotrexate or intolerant to the drug, biologic therapies, an alternate disease-modifying antirheumatic drug (DMARD) monotherapy, or a combination of these treatments. Methotrexate has been used as an alternative to corticosteroids in the treatment of chronic and refractory sarcoidosis for years. It has proven to effectively manage the disease in many patients.63 However, no studies have looked at its role in sarcoid arthritis in particular. Its benefits to patients with rheumatoid arthritis have been well documented over the past 40 years, and it is therefore considered a first-line therapy in that disease setting.64 Despite the clinical utility of DMARDs, randomized studies for some (e.g., sulfasalazine, hydroxychloroquine, leflunamide, azathioprine, and minocycline) are lacking in the sarcoidosis setting. Even in the context of rheumatoid arthritis, the evidence for sulfasalazine and minocycline benefit comes from case reports, observational studies, and small nonrandomized studies.65 Keeping in mind that there is a lack of large, randomized trials evaluating the utility of DMARDs in sarcoidosis, our suggested treatment algorithm proposes these agents in the first-line setting for some patients, based on our own clinical experience. Of note, clinicians should monitor patients’ progress and periodically check for toxicities (Table 1). An approach for toxicity monitoring prior to initiating therapy or increasing dosages of DMARDs was recently proposed by the American College of Rheumatology based on the rheumatoid arthritis literature. As shown in Table 1, baseline evaluations of patients receiving DMARDs should include complete blood count (CBC), liver transaminase, creatinine, hepatitis B and C testing, and ophthalmologic evaluations.65 For patients failing second-line treatment, a different biologic agent, a trial of high-dose systemic steroids, or enrollment in a clinical trial should be considered.
Figure 3.
iagnosis and management of patients with sarcoid arthritis. MTX, methotrexate; SZA, sulfasalazine; DMARD, disease modifying antirheumatic drugs (hydroxychloroquine, MTX, SZA, minocycline, azathioprine, leflunomide); NSAIDs, nonsteroidal antiinflammatory drugs; PT/OT, physical therapy/occupational therapy; biologics, anti-TNF agents (Infliximab, adalimumab), co-stimulatory agents (abatacept); B-cell depleting agents (rituximab); combination therapy, multiple DMARDs and/or a DMARD plus a biologic. *Low-dose steroids, <10 to 20 mg prednisone daily **Suboptimal response to MTX, intolerance to drug, lack of satisfactory efficacy on dosage up to 25 mg/week, or a contraindication to medication use. ***DMARD failure, progressive disease or drug intolerance. ****Methylprednisone preferred over prednisone if prednisone has been used prior.
Table 1.
Recommendations on Baseline Evaluation for Starting, Resuming, or Significant Dose Increase of a Therapy in Patients with Rheumatoid Arthritis Receiving Nonbiologic and Biologic Disease-Modifying Antirheumatic Drugs*
| Therapeutic Agents | CBC† | Liver Transaminase |
Creatinine | Hepatitis B and C Testing‡ |
Ophthalmologic Examination‡ |
|---|---|---|---|---|---|
| Hydroxychloroquine | X | X | X | X | |
| Leflunomide | X | X | X | X | |
| Methotrexate | X | X | X | X | |
| Minocycline | X | X | X | ||
| Sulfasalazine | X | X | X | ||
| All biologic agents | X | X | X |
Reproduced with permission from Saag et al.65
CBC, complete blood count.
If hepatitis risk factors are present (e.g., intravenous drug abuse, multiple sex partners in the previous 6 months, health care personnel). Evaluation might include tests for hepatitis B surface antigen, hepatitis B antibodies, hepatitis B core antibodies, and/or hepatitis C antibodies.
Ophthalmologic examination is recommended within the first year of treatment. For patients in higher-risk categories (e.g., liver disease, concomitant retinal disease, and age ≥60 years), the American Academy of Ophthalmology recommends an annual follow-up eye examination.
CASE STUDIES
Case 1
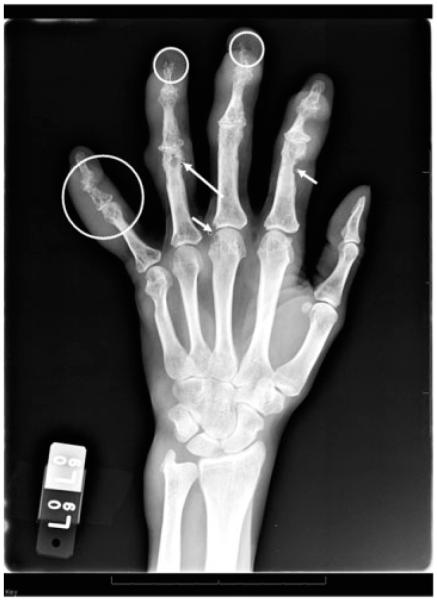
A 47-year-old African American woman with long-standing biopsy-proven sarcoidosis, presented with progressive arthritis involving pain in multiple finger joints and left shoulder, dyspnea on exertion, and prior hemoptysis. The disease was complicated by stage IV fibrocavitary lung disease, lung mycetoma, and traction bronchiectasis, which required antifungal therapy. The arthritis was minimally responsive to NSAIDs, methotrexate, and low-dose prednisone (4 mg). Rheumatologic examination showed synovitis and erythemas of the distal interpharyngeal (DIP) joints and the proximal interpharyngeal (PIP) joints bilaterally. Dystrophic nail changes were also noted. Photographs of the same hands are shown in Fig. 4 and reveal diffuse swelling involving the PIP and DIP joints. Erythema is also noted at both joints; at the DIP, indicating that the sarcoid arthritis is acute, and at the PIP, suggesting that the arthritis is chronic. Dystrophic nail changes are also noted, and there is no evidence of pitting. Hand radiographs shown in Fig. 5 reveal osseous erosions bilaterally. Lacy reticular patterns, which are characteristic of sarcoidosis and acroosteolysis of distal phalanx tufts are shown as well as granulomatous erosions (Fig. 5). Methylprednisolone at a dose of 8 mg/day failed to control the signs and symptoms of arthritis; injectable methotrexate at a dose of 10 mg/week was added due to poor tolerance to oral methotrexate. The combination of methylprednisone and methotrexate resulted in partial improvement of the arthritis signs and symptoms. Hydroxychloroquine at a dose of 400 mg daily was added. Anti-TNF-α therapy was not initiated given the active fungal infection.
Figure 4.
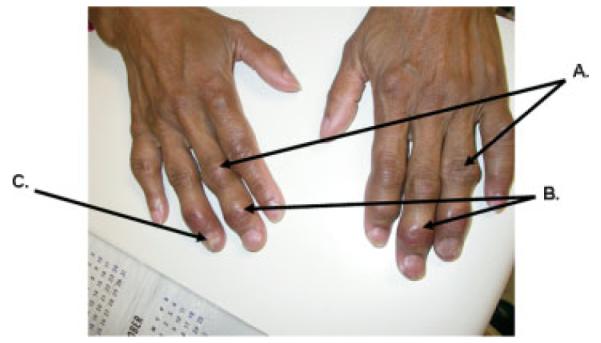
and photograph shows (A) proximal interpharyngeal (PIP) joint swelling and erythema, (B) distal interpharyngeal (DIP) joint swelling and erythema, and (C) dystrophic nail changes, without evidence of pitting.
Figure 5.
and x-ray from patient in Figure 4 reveals osseous erosions bilaterally. Big circle: Lacy reticular pattern characteristic of sarcoidosis—fifth digit and soft tissue swelling. Small circles: Acroosteolysis of distal phalanx tufts. Long arrow: Punched-out granuloma neck proximal phalanx of ring finger. Short arrows: Granulomatous erosion.
Case 2
A 42-year-old healthy Caucasian man presented with several years’ history of back pain. Spine radiographs showed a pathological L4 compression fracture as well as multiple vertebral hypodense lesions as well as iliac crest and humorous lesions (Fig. 6). A diagnosis of sarcoidosis was made after a thorough workup for malignancy. Open bone biopsy from the left humerus and the left iliac crest showed noncaseating granulomas, acid-fast bacilli stains, and fungal stains; cultures were negative. The disease was complicated by osteoporosis that was treated with oral bisphosphonates, but there was no evidence of metabolic bone disease, and serum calcium was within normal limits. There was no evidence of lung, hepatic, cardiac, or neurosarcoidosis. Oral prednisone at a dose of 10 mg/day for a few months resulted in partial pain control. His symptoms returned when the prednisone dose was tapered to 5 mg/day. Oral prednisone was switched to methyl-prednisolone at a dose of 4 mg/day with significant symptomatic improvement. Repeat spine images with radiographs and MRI showed stabilization of some lesions and improvement in others. Hydroxychloroquine was added as a steroid-sparing agent due to the patient’s history of osteoporosis.
Figure 6.
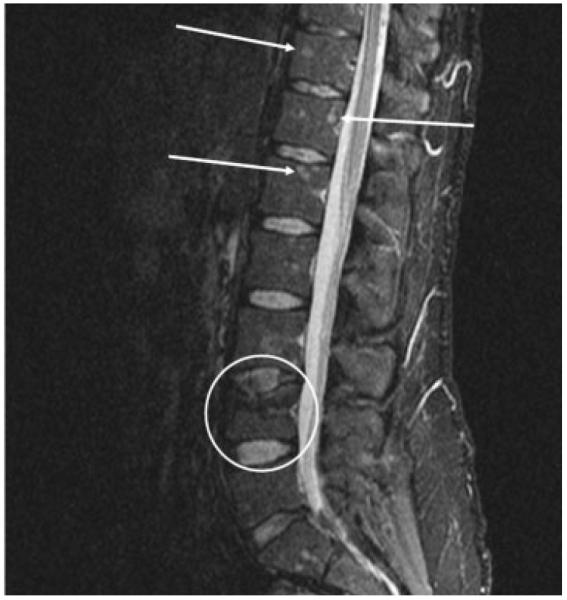
pine magnetic resonance imaging without contrast STIR (short tau inversion recovery), sagittal view demonstrating axial sarcoid disease. Circle indicates pathological L4 compression fracture. Arrows indicate multiple sarcoid bony lesions.
DISCUSSION
There is a need for well-designed clinical trials to further aid in the generation of a standardized approach to the treatment of all variations of rheumatic sarcoid. The optimal dose, type, and route of administration of corticosteroids are unknown. Typically the choice of medication use depends on what is required for other organ involvement in sarcoid, as well as on the treating physician’s experience with corticosteroid-sparing agents. We support that the use of corticosteroid-sparing agents as well as biologics, while at times successful, should be considered investigational and should only be tried after thorough discussion with patients.
Additional future studies employing high throughput strategies such as genomewide expression studies and GWASs will likely push the field ahead and provide clinicians with a better understanding of the complex nature of this fascinating disease as well as identify novel targets for disease amelioration by targeted therapies. For example, given the role of TNF-α in sarcoidosis, use of TNF-α inhibitors is steadily increasing. Recently, these agents have been shown to improve index organ involvement in patients with refractory sarcoidosis.17,66–69 However, drug-induced sarcoidosis in nonsarcoid patients treated with TNF-α inhibitors has been increasingly reported, and patients often relapse after discontinuation of therapy with these agents.70–73 Although the potential mechanism for the induction of sarcoidosis by TNF-α inhibitors is not clear, there are interesting data supporting a cross-regulation of interferon (IFN-α) by TNF-α in humans,74,75 including some studies in which TNF-α blockade results in increased IFN-α.76 This is of interest because there are a number of cases of sarcoidosis and other autoimmune conditions induced by exogenous IFN-α given as a treatment.77,78 It is possible that in some individuals, TNF-α blockade results in significant dysregulation of IFN-α and subsequent induction of sarcoidosis.
The use of B cell–depleting agents may also be of benefit in sarcoid arthritis. B cell–depleting agents are used with success in patients with rheumatoid arthritis who failed anti-TNF-α therapy. A recently published case report of a patient with sarcoidosis of the lungs and joints reported that the typical rheumatoid arthritis schedule of rituximab was effective in treating the sarcoidosis with no major side effects. However, the duration of symptom improvement was only 1 year.79 Given the success of rituximab, other B cell therapies such as ocrelizumab, have also been evaluated in rheumatoid arthritis and have shown clinical efficacy and safety. More studies are warranted to further characterize the role of rituximab and ocrelizumab in rheumatoid arthritis and other autoimmune diseases, such as systemic sarcoidosis.80 Other agents, abatacept and tocilizumab, have been successfully used in patients with rheumatoid arthritis. In the future, they could also potentially be used in patients with rheumatic manifestations of sarcoidosis.
Multidisciplinary approaches that incorporate translational research are required to address the many complex questions that remain in sarcoidosis research. Until better evidence is available from multicenter randomized trials, treating patients with rheumatic manifestations of sarcoidosis needs to be individualized and should take into consideration the multisystem nature of the disease and its comorbidities. A unified team approach is needed to serve our patients best.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Orlin Hadjiev, University of Chicago, and Dr. Cheng Hong, Universityof Chicago, for their contributions. In addition, we thank Kelly McCoy for her editorial assistance in preparing this manuscript.
REFERENCES
- 1.Hofmann S, Franke A, Fischer A, et al. Genome-wide association study identifies ANXA11 as a new susceptibility locus for sarcoidosis. Nat Genet. 2008;40:1103–1106. doi: 10.1038/ng.198. [DOI] [PubMed] [Google Scholar]
- 2.Chen ES, Moller DR. Etiology of sarcoidosis. Clin Chest Med. 2008;29:365–377. vii. doi: 10.1016/j.ccm.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 3.Smith G, Brownell I, Sanchez M, Prystowsky S. Advances in the genetics of sarcoidosis. Clin Genet. 2008;73:401–412. doi: 10.1111/j.1399-0004.2008.00970.x. [DOI] [PubMed] [Google Scholar]
- 4.Drake WP, Newman LS. Mycobacterial antigens may be important in sarcoidosis pathogenesis. Curr Opin Pulm Med. 2006;12:359–363. doi: 10.1097/01.mcp.0000239554.01068.94. [DOI] [PubMed] [Google Scholar]
- 5.Ezzie ME, Crouser ED. Considering an infectious etiology of sarcoidosis. Clin Dermatol. 2007;25:259–266. doi: 10.1016/j.clindermatol.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Maples CJ, Counselman FL. Lupus pernio. J Emerg Med. 2007;33:187–189. doi: 10.1016/j.jemermed.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 7.Wilcox A, Bharadwaj P, Sharma OP. Bone sarcoidosis. Curr Opin Rheumatol. 2000;12:321–330. doi: 10.1097/00002281-200007000-00016. [DOI] [PubMed] [Google Scholar]
- 8.Bargagli E, Olivieri C, Penza F, et al. Rare localizations of bone sarcoidosis: two case reports and review of the literature. Rheumatol Int. 2009 Dec. 15 doi: 10.1007/s00296-009-1315-7. [DOI] [PubMed] [Google Scholar]
- 9.Chatham W. Rheumatic manifestations of systemic disease: sarcoidosis. Curr Opin Rheumatol. 2010;22:85–90. doi: 10.1097/BOR.0b013e328333ba74. [DOI] [PubMed] [Google Scholar]
- 10.Ugwonali OF, Parisien M, Nickerson KG, Scully B, Ristic S, Strauch RJ. Osseous sarcoidosis of the hand: pathologic analysis and review of the literature. J Hand Surg [Am] 2005;30:854–858. doi: 10.1016/j.jhsa.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Shorr AF, Murphy FT, Kelly WF, Kaplan KJ, Gilliland WR, Shapeero LG. Osseous sarcoidosis clinical, radiographic, and therapeutic observations. J Clin Rheumatol. 1998;4:186–192. doi: 10.1097/00124743-199808000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Shorr AF, Murphy FT, Gilliland WR, Hnatiuk W. Osseous disease in patients with pulmonary sarcoidosis and musculoskeletal symptoms. Respir Med. 2000;94:228–232. doi: 10.1053/rmed.1999.0709. [DOI] [PubMed] [Google Scholar]
- 13.Rúa-Figueroa I, Gantes MA, Erausquin C, Mhaidli H, Montesdeoca A. Vertebral sarcoidosis: clinical and imaging findings. Semin Arthritis Rheum. 2002;31:346–352. doi: 10.1053/sarh.2002.31553. [DOI] [PubMed] [Google Scholar]
- 14.Binicier O, Sari I, Sen G, et al. Axial sarcoidosis mimicking radiographic sacroiliitis. Rheumatol Int. 2009;29:343–345. doi: 10.1007/s00296-008-0677-6. [DOI] [PubMed] [Google Scholar]
- 15.Moore SL, Teirstein AE. Musculoskeletal sarcoidosis: spectrum of appearances at MR imaging. Radiographics. 2003;23:1389–1399. doi: 10.1148/rg.236025172. [DOI] [PubMed] [Google Scholar]
- 16.Mangino D, Stover DE. Sarcoidosis presenting as metastatic bony disease: a case report and review of the literature on vertebral body sarcoidosis. Respiration. 2004;71:292–294. doi: 10.1159/000077430. [DOI] [PubMed] [Google Scholar]
- 17.Garg S, Garg K, Altaf M, Magaldi JA. Refractory vertebral sarcoidosis responding to infliximab. J Clin Rheumatol. 2008;14:238–240. doi: 10.1097/RHU.0b013e318181b45a. [DOI] [PubMed] [Google Scholar]
- 18.Kötter I, Dürk H, Saal JG. Sacroiliitis in sarcoidosis: case reports and review of the literature. Clin Rheumatol. 1995;14:695–700. doi: 10.1007/BF02207939. [DOI] [PubMed] [Google Scholar]
- 19.Erb N, Cushley MJ, Kassimos DG, Shave RM, Kitas GD. An assessment of back pain and the prevalence of sacroiliitis in sarcoidosis. Chest. 2005;127:192–196. doi: 10.1378/chest.127.1.192. [DOI] [PubMed] [Google Scholar]
- 20.Griep EN, van Spiegel PI, van Soesbergen RM. Sarcoidosis accompanied by pulmonary tuberculosis and complicated by sacroiliitis. Arthritis Rheum. 1993;36:716–721. doi: 10.1002/art.1780360521. [DOI] [PubMed] [Google Scholar]
- 21.Zisman DA, Biermann JS, Martinez FJ, Devaney KO, Lynch JP., III Sarcoidosis presenting as a tumorlike muscular lesion: case report and review of the literature. Medicine (Baltimore) 1999;78:112–122. doi: 10.1097/00005792-199903000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Moore SL, Teirstein AE, Tohme-Noun C, et al. Musculoskeletal sarcoidosis: spectrum of appearances at MR imaging. Radiographics. 2003;23:1389–1399. doi: 10.1148/rg.236025172. [DOI] [PubMed] [Google Scholar]
- 23.Torralba KD, Quismorio FP., Jr Sarcoidosis and the rheumatologist. Curr Opin Rheumatol. 2009;21:62–70. doi: 10.1097/bor.0b013e32831dde88. [DOI] [PubMed] [Google Scholar]
- 24.Nemoto I, Shimizu T, Fujita Y, Tateishi Y, Tsuji-Abe Y, Shimizu H. Tumour-like muscular sarcoidosis. Clin Exp Dermatol. 2007;32:298–300. doi: 10.1111/j.1365-2230.2007.02371.x. [DOI] [PubMed] [Google Scholar]
- 25.Le Roux K, Streichenberger N, Vial C, et al. Granulomatous myositis: a clinical study of thirteen cases. Muscle Nerve. 2007;35:171–177. doi: 10.1002/mus.20683. [DOI] [PubMed] [Google Scholar]
- 26.Matsui K, Adachi M, Kawasaki Y, Matsuda K, Shinohara K. Sarcoidosis acutely involving the musculoskeletal system. Intern Med. 2007;46:1471–1474. doi: 10.2169/internalmedicine.46.6375. [DOI] [PubMed] [Google Scholar]
- 27.Fayad F, Lioté F, Berenbaum F, Orcel P, Bardin T. Muscle involvement in sarcoidosis: a retrospective and followup studies. J Rheumatol. 2006;33:98–103. [PubMed] [Google Scholar]
- 28.Sève P, Zénone T, Durieu I, Pillon D, Durand DV. Muscular sarcoidosis: apropos of a case [in French] Rev Med Interne. 1997;18:984–988. doi: 10.1016/s0248-8663(97)80120-8. [DOI] [PubMed] [Google Scholar]
- 29.Danon MJ, Perurena OH, Ronan S, Manaligod JR. Inclusion body myositis associated with systemic sarcoidosis. Can J Neurol Sci. 1986;13:334–336. doi: 10.1017/s0317167100036684. [DOI] [PubMed] [Google Scholar]
- 30.Vattemi G, Tonin P, Marini M, et al. Sarcoidosis and inclusion body myositis. Rheumatology (Oxford) 2008;47:1433–1435. doi: 10.1093/rheumatology/ken252. [DOI] [PubMed] [Google Scholar]
- 30a.Larue S, Maisonobe T, Benveniste O, et al. Distal muscle involvement in granulomatous myositis can mimic inclusion body myositis. J Neurol Neurosurg Psychatry. 2010 June 20; doi: 10.1136/jnnp.2009.190751. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 31.Fernandes SR, Singsen BH, Hoffman GS. Sarcoidosis and systemic vasculitis. Semin Arthritis Rheum. 2000;30:33–46. doi: 10.1053/sarh.2000.8364. [DOI] [PubMed] [Google Scholar]
- 32.Stone J. Rituximab versus cyclophosphamide for induction of remission in ANCA-associated vasculitis: a randomized controlled trial (RAVE). Arthritis & Rheumatism; American College of Rheumatology Conference; Philadelphia, PA. October 16–21, 2009; 2009. abstract 550. [Google Scholar]
- 33.Mansour MJ, Al-Hashimi I, Wright JM. Coexistence of Sjögren’s syndrome and sarcoidosis: a report of five cases. J Oral Pathol Med. 2007;36:337–341. doi: 10.1111/j.1600-0714.2007.00530.x. [DOI] [PubMed] [Google Scholar]
- 34.Rudralingam M, Nolan A, Macleod I, Greenwood M, Heath N. A case of sarcoidosis presenting with diffuse, bilateral swelling of the salivary glands. Dent Update. 2007;34:439–440. 442. doi: 10.12968/denu.2007.34.7.439. [DOI] [PubMed] [Google Scholar]
- 35.Hansen SR, Hetta AK, Omdal R. Primary Sjögren’s syndrome and sarcoidosis: coexistence more than by chance? Scand J Rheumatol. 2008;37:485–486. doi: 10.1080/03009740802220075. [DOI] [PubMed] [Google Scholar]
- 36.van de Loosdrecht A, Kalk W, Bootsma H, Henselmans JM, Kraan J, Kallenberg CG. Simultaneous presentation of sarcoidosis and Sjögren’s syndrome. Rheumatology (Oxford) 2001;40:113–115. doi: 10.1093/rheumatology/40.1.113. [DOI] [PubMed] [Google Scholar]
- 37.Bamba R, Sweiss NJ, Langerman AJ, Taxy JB, Blair EA. The minor salivary gland biopsy as a diagnostic tool for Sjogren syndrome. Laryngoscope. 2009;119:1922–1926. doi: 10.1002/lary.20292. [DOI] [PubMed] [Google Scholar]
- 38.Stonecipher K, Perry HD, Gross RH, Kerney DL. The impact of topical cyclosporine A emulsion 0.05% on the outcomes of patients with keratoconjunctivitis sicca. Curr Med Res Opin. 2005;21:1057–1063. doi: 10.1185/030079905X50615. [DOI] [PubMed] [Google Scholar]
- 39.Perruquet JL, Harrington TM, Davis DE, Viozzi FJ. Sarcoid arthritis in a North American Caucasian population. J Rheumatol. 1984;11:521–525. [PubMed] [Google Scholar]
- 40.Pettersson T. Sarcoid and erythema nodosum arthropathies. Best Pract Res Clin Rheumatol. 2000;14:461–476. doi: 10.1053/berh.2000.0088. [DOI] [PubMed] [Google Scholar]
- 41.Spilberg I, Siltzbach LE, McEwen C. The arthritis of sarcoidosis. Arthritis Rheum. 1969;12:126–137. doi: 10.1002/art.1780120209. [DOI] [PubMed] [Google Scholar]
- 42.Visser H, Vos K, Zanelli E, et al. Sarcoid arthritis: clinical characteristics, diagnostic aspects, and risk factors. Ann Rheum Dis. 2002;61:499–504. doi: 10.1136/ard.61.6.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ohta H, Tazawa R, Nakamura A, et al. Acute-onset sarcoidosis with erythema nodosum and polyarthralgia (Löfgren’s syndrome) in Japan: a case report and a review of the literature. Intern Med. 2006;45:659–662. doi: 10.2169/internalmedicine.45.1452. [DOI] [PubMed] [Google Scholar]
- 44.Sato T, Tsuru T, Hagiwara K, et al. Sarcoidosis with acute recurrent polyarthritis and hypercalcemia. Intern Med. 2006;45:363–368. doi: 10.2169/internalmedicine.45.1521. [DOI] [PubMed] [Google Scholar]
- 45.Chu A, Ginat D, Terzakis J, Seneviratne A, Schneider KS. Chronic sarcoid arthritis presenting as an intra-articular knee mass. J Clin Rheumatol. 2009;15:190–192. doi: 10.1097/RHU.0b013e3181a61c29. [DOI] [PubMed] [Google Scholar]
- 46.Glennås A, Kvien TK, Melby K, et al. Acute sarcoid arthritis: occurrence, seasonal onset, clinical features and outcome. Br J Rheumatol. 1995;34:45–50. doi: 10.1093/rheumatology/34.1.45. [DOI] [PubMed] [Google Scholar]
- 47.Gran JT, Bøhmer E. Acute sarcoid arthritis: a favourable outcome? A retrospective survey of 49 patients with review of the literature. Scand J Rheumatol. 1996;25:70–73. doi: 10.3109/03009749609069210. [DOI] [PubMed] [Google Scholar]
- 48.Grunewald J, Eklund A. Role of CD4 T cells in sarcoidosis. Proc Am Thorac Soc. 2007;4:461–464. doi: 10.1513/pats.200606-130MS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thelier N, Assous N, Job-Deslandre C, et al. Osteoarticular involvement in a series of 100 patients with sarcoidosis referred to rheumatology departments. J Rheumatol. 2008;35:1622–1628. [PubMed] [Google Scholar]
- 50.Neville E, Walker AN, James DG. Prognostic factors predicting the outcome of sarcoidosis: an analysis of 818 patients. Q J Med. 1983;52:525–533. [PubMed] [Google Scholar]
- 51.Pennec Y, Youinou P, Le Goff P, Boles JM, Le Menn G. Comparison of the manifestations of acute sarcoid arthritis with and without erythema nodosum. Immunopathogenic significance. Scand J Rheumatol. 1982;11:13–16. doi: 10.3109/03009748209098106. [DOI] [PubMed] [Google Scholar]
- 52.Anandacoomarasamy A, Peduto A, Howe G, Manolios N, Spencer D. Magnetic resonance imaging in Löfgren’s syndrome: demonstration of periarthritis. Clin Rheumatol. 2007;26:572–575. doi: 10.1007/s10067-006-0360-9. [DOI] [PubMed] [Google Scholar]
- 53.Kellner H, Späthling S, Herzer P. Ultrasound findings in Löfgren’s syndrome: is ankle swelling caused by arthritis, tenosynovitis or periarthritis? J Rheumatol. 1992;19:38–41. [PubMed] [Google Scholar]
- 54.Proulx AM, Zryd TW. Costochondritis: diagnosis and treatment. Am Fam Physician. 2009;80:617–620. [PubMed] [Google Scholar]
- 55.Baughman RP, Iannuzzi MC. Diagnosis of sarcoidosis: when is a peek good enough? Chest. 2000;117:931–932. doi: 10.1378/chest.117.4.931. [DOI] [PubMed] [Google Scholar]
- 56.Kaplan H, Klatskin G. Sarcoidosis, psoriasis, and gout: syndrome or coincidence? Yale J Biol Med. 1960;32:335–352. [PMC free article] [PubMed] [Google Scholar]
- 57.Goldstein RA, Becker KL, Israel HL, Moore CF. Urate metabolism in sarcoidosis. Arch Intern Med. 1974;133:379–381. [PubMed] [Google Scholar]
- 58.Longcope WT, Freiman DG. A study of sarcoidosis; based on a combined investigation of 160 cases including 30 autopsies from The Johns Hopkins Hospital and Massachusetts General Hospital. Medicine (Baltimore) 1952;31:1–132. [PubMed] [Google Scholar]
- 59.Healy PJ, Helliwell PS. Dactylitis: pathogenesis and clinical considerations. Curr Rheumatol Rep. 2006;8:338–341. doi: 10.1007/s11926-006-0062-y. [DOI] [PubMed] [Google Scholar]
- 60.Rothschild BM, Pingitore C, Eaton M. Dactylitis: implications for clinical practice. Semin Arthritis Rheum. 1998;28:41–47. doi: 10.1016/s0049-0172(98)80027-9. [DOI] [PubMed] [Google Scholar]
- 61.Kircik LH, Del Rosso JQ. Anti-TNF agents for the treatment of psoriasis. J Drugs Dermatol. 2009;8:546–559. [PubMed] [Google Scholar]
- 62.Cuchacovich R, Espinoza CG, Virk Z, Espinoza LR. Biologic therapy (TNF-alpha antagonists)-induced psoriasis: a cytokine imbalance between TNF-alpha and IFN-alpha? J Clin Rheumatol. 2008;14:353–356. doi: 10.1097/RHU.0b013e318190dd88. [DOI] [PubMed] [Google Scholar]
- 63.Baughman RP, Lower EE. A clinical approach to the use of methotrexate for sarcoidosis. Thorax. 1999;54:742–746. doi: 10.1136/thx.54.8.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Braun J, Rau R. An update on methotrexate. Curr Opin Rheumatol. 2009;21:216–223. doi: 10.1097/BOR.0b013e328329c79d. [DOI] [PubMed] [Google Scholar]
- 65.Saag KG, Teng GG, Patkar NM, et al. American College of Rheumatology American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008;59:762–784. doi: 10.1002/art.23721. [DOI] [PubMed] [Google Scholar]
- 66.Moravan M, Segal BM. Treatment of CNS sarcoidosis with infliximab and mycophenolate mofetil. Neurology. 2009;72:337–340. doi: 10.1212/01.wnl.0000341278.26993.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Agrawal S, Bhagat S, Dasgupta B. Sarcoid sacroiliitis: successful treatment with infliximab. Ann Rheum Dis. 2009;68:283. doi: 10.1136/ard.2007.087155. [DOI] [PubMed] [Google Scholar]
- 68.Santos E, Shaunak S, Renowden SA, Scolding NJ. Treatment of refractory neurosarcoidosis with infliximab. J Neurol Neurosurg Psychiatry. 2010;81:241–246. doi: 10.1136/jnnp.2008.149989. [DOI] [PubMed] [Google Scholar]
- 69.Barnabe C, McMeekin J, Howarth A, Martin L. Successful treatment of cardiac sarcoidosis with infliximab. J Rheumatol. 2008;35:1686–1687. [PubMed] [Google Scholar]
- 70.Sweiss NJ, Baughman RP. Tumor necrosis factor inhibition in the treatment of refractory sarcoidosis: slaying the dragon? J Rheumatol. 2007;34:2129–2131. [PubMed] [Google Scholar]
- 71.Sweiss NJ, Curran J, Baughman RP. Sarcoidosis, role of tumor necrosis factor inhibitors and other biologic agents, past, present, and future concepts. Clin Dermatol. 2007;25:341–346. doi: 10.1016/j.clindermatol.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 72.Sweiss NJ, Hushaw LL. Biologic agents for rheumatoid arthritis: 2008 and beyond. J Infus Nurs. 2009;32(1 Suppl):S4–17. doi: 10.1097/NAN.0b013e318192e311. quiz S19–24. [DOI] [PubMed] [Google Scholar]
- 73.Sweiss NJ, Welsch MJ, Curran JJ, Ellman MH. Tumor necrosis factor inhibition as a novel treatment for refractory sarcoidosis. Arthritis Rheum. 2005;53:788–791. doi: 10.1002/art.21468. [DOI] [PubMed] [Google Scholar]
- 74.Palucka AK, Blanck JP, Bennett L, Pascual V, Banchereau J. Cross-regulation of TNF and IFN-alpha in autoimmune diseases. Proc Natl Acad Sci U S A. 2005;102:3372–3377. doi: 10.1073/pnas.0408506102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kariuki SN, Crow MK, Niewold TB. The PTPN22 C1858T polymorphism is associated with skewing of cytokine profiles toward high interferon-alpha activity and low tumor necrosis factor alpha levels in patients with lupus. Arthritis Rheum. 2008;58:2818–2823. doi: 10.1002/art.23728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mavragani CP, Niewold TB, Moutsopoulos NM, Pillemer SR, Wahl SM, Crow MK. Augmented interferon-alpha pathway activation in patients with Sjögren’s syndrome treated with etanercept. Arthritis Rheum. 2007;56:3995–4004. doi: 10.1002/art.23062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Doyle MK, Berggren R, Magnus JH. Interferon-induced sarcoidosis. J Clin Rheumatol. 2006;12:241–248. doi: 10.1097/01.rhu.0000240035.67652.9d. [DOI] [PubMed] [Google Scholar]
- 78.Niewold TB. Interferon alpha-induced lupus: proof of principle. J Clin Rheumatol. 2008;14:131–132. doi: 10.1097/RHU.0b013e318177627d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Belkhou A, Younsi R, El Bouchti I, El Hassani S. Rituximab as a treatment alternative in sarcoidosis. Joint Bone Spine. 2008;75:511–512. doi: 10.1016/j.jbspin.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 80.Kausar F, Mustafa K, Sweis G, et al. Ocrelizumab: a step forward in the evolution of B-cell therapy. Expert Opin Biol Ther. 2009;9:889–895. doi: 10.1517/14712590903018837. [DOI] [PubMed] [Google Scholar]