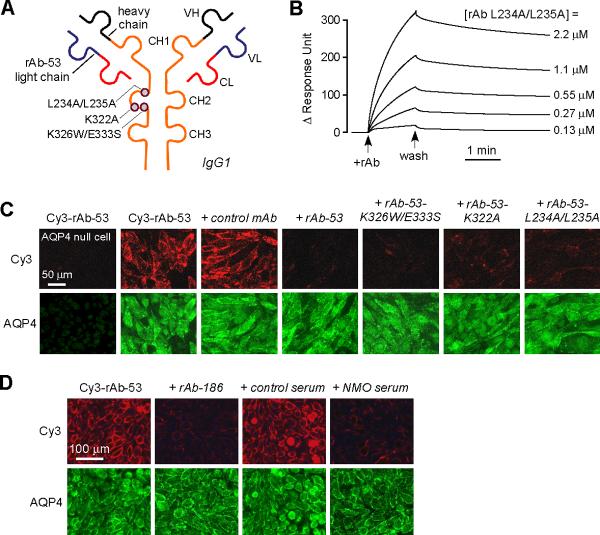
Figure 2. Mutated, non-pathogenic rAb-53 monoclonal antibodies blocks binding of pathogenic NMO-IgG to AQP4.
a. Schematic of rAb-53 showing heavy (VH) and light (VL) chain variable regions, light chain constant region (CL), and IgG1 heavy chain constant regions (CH1-CH3). Locations of amino acid mutations introduced in the CH2 domain to reduce CDC (K322A), ADCC (K326W/E333S) or both (L234A/L235A). b. Surface plasmon resonance measurements of binding and washout of a mutated rAb-53 (L234A/L235A) to AQP4-reconstituted proteoliposomes. c. Mutated rAb-53 blocks binding of Cy3-labeled (non-mutated) rAb-53 to AQP4-expressing cells. Cy3 fluorescence imaged in AQP4-null (left-most panel) or AQP4-expressing (other panels) cells incubated with 20 μg/ml Cy3-rAb-53 for 1 h in the absence or presence of indicated (unlabeled) antibodies at 100 μg/ml. d. Unrelated monoclonal NMO antibodies and human NMO serum block AQP4 binding of Cy3-labeled rAb-53. Cy3 fluorescence imaged in cells incubated with 20 μg/ml Cy3-rAb-53 for 1 h in the absence or presence of 10% control (non-NMO) or NMO patient serum, or 100 μg/ml recombinant NMO monoclonal antibody rAb-186.