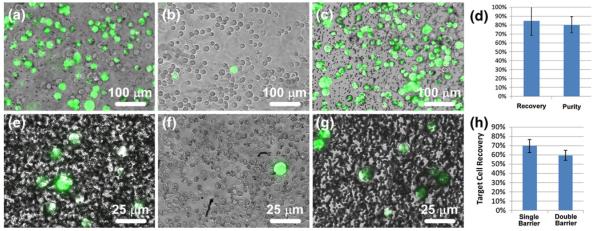
Fig. 4.
Separation of MCF-7 cells (green) from HS-5 cells (uncolored) and whole human blood. An initial mixture (a) containing approximately 43% MCF-7 cells and 57% HS-5 cells was sorted via single barrier IFAST and the numbers of each cell type in the input and output wells were counted. The majority (average of 89%) of HS-5 cells were left in the input well (b) while most (average of 85%) of the MCF-7 cells were transferred to the output well (c). (d) The recovery level of the sorted MCF-7 cells and purity of the output well are similar to values observed in the characterization experiments. When MCF-7 cells were sorted from whole blood using a single barrier IFAST device, a significant number of blood cells were transferred to the output well (e). When using a double barrier IFAST device, the majority of these cells were released in the middle well ((f), a single MCF-7 cell surrounded primarily by red blood cells), resulting in a relatively pure sample in the output well (g). When a second oil traverse was added, recovery was reduced from 70% to 59% (h). Due to the low concentration of MCF-7 cells, lots of unbound PMPs are seen in Image E and G. (Note the dark dots in (g) are unbound PMPs, not contaminant cells.) Images A-C were obtained using a 10× objective while images E-G were obtained with a 40× objective. The input wells of IFAST devices used for the blood sorting experiments were too densely populated with blood cells to image