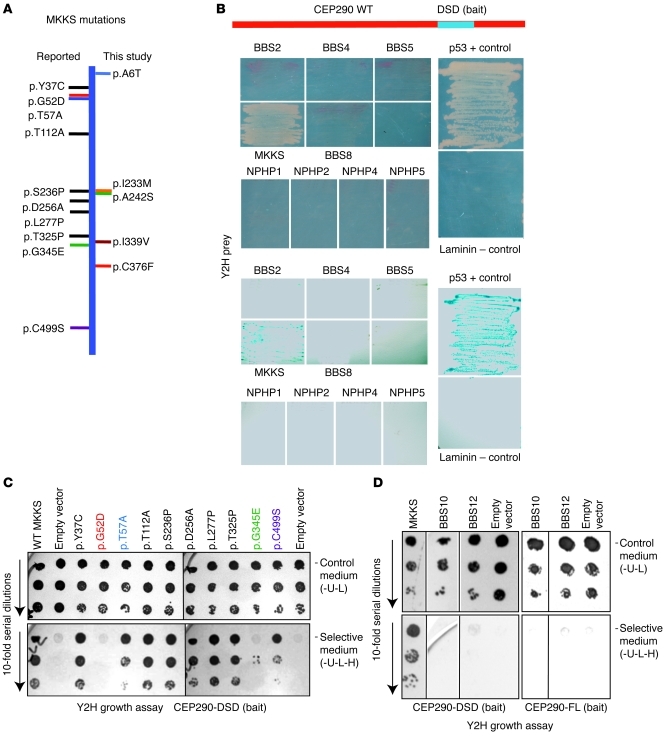
Figure 1. The CEP290-DSD domain specifically interacts with MKKS.
(A) A schematic of the MKKS protein, with horizontal lines showing variants/mutations identified in this study (right) and previously reported (left). New sequence variants cluster in the same regions as known mutations. (B) Yeast 2-hybrid (Y2H) screen using the Cep290-DSD domain as bait identified MKKS as a specific interactor. p53/T-antigen and laminin/T-antigen combinations were positive and negative controls, respectively. Growth was detected with the MKKS prey only (top panels) and validated by β-galactosidase assay (bottom panels). (C) Interaction of the Cep290-DSD domain with mutant MKKS constructs in yeast 2-hybrid growth assay. The top 3 rows show growth of 10-fold serial cell dilutions on double-selection control media (-U-L); the bottom 3 rows show growth on selective media (-U-L-H). Note equal growth on control media plates (top 3 rows). MKKS mutations at residues 52, 57, 345, and 499 disrupt interaction with CEP290. (D) Yeast 2-hybrid assay using CEP290-DSD (left 4 lanes) or full-length CEP290 (CEP290-FL) (right 3 lanes) as bait with full-length MKKS, BBS10, BBS12, or empty vector as prey. The top 3 rows show growth of 10-fold serial cell dilutions on double-selection control media (-U-L); the bottom 3 rows show growth of 10-fold serial dilutions on selective media (-U-L-H). Only MKKS interacts with Cep290-DSD. The first row of the BBS12 lane on selective medium shows a shadow due to dead cells.