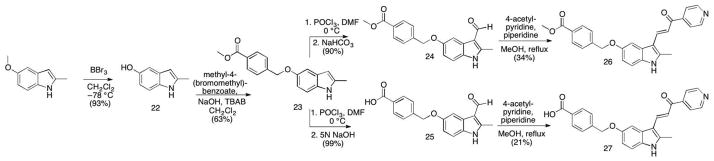
Scheme 2.
Analogs with 5′ modifications of the indole ring generated by functionalizing the indole prior to introduction of the pyridine moiety. Conditions and reagents: (compound 22) BBr3, CH2Cl2, – 78 °C, 93%; (compound 23) methyl-4-(bromomethyl)benzoate, NaOH, TBAB, CH2Cl2, RT, 63%; (compound 24) 1. POCl3, DMF, 0 °C; 2. NaHCO3, 90%; (compound 25) 1. POCl3, DMF, 0 °C; 2. 5N NaOH, 99%; (compound 26) 4-acetyl-pyridine, piperidine, MeOH, reflux, 34%; (compound 27) 4-acetyl-pyridine, piperidine, MeOH, reflux, 21%.