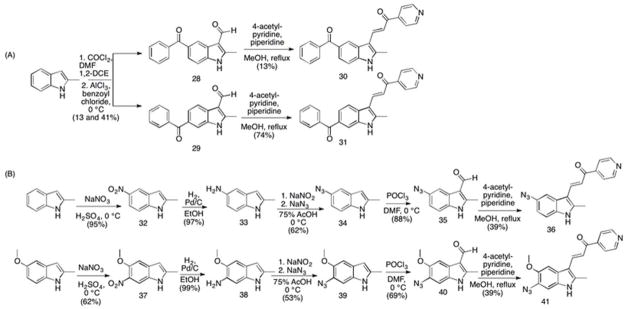
Scheme 3.
Synthesis of 5′ and 6′ azido derivatives of MIPP. (A) Conditions and reagents: (compounds 28 & 29) 1. COCl2, DMF, 1,2-DCE, 0 °C; 2. AlCl3, benzoyl chloride, 0 °C RT, 13% and 41% for 28 and 29, respectively; (compound 30) 4-acetyl-pyridine, piperidine, MeOH, reflux, 13%; (compound 31) 4-acetyl-pyridine, piperidine, MeOH, reflux, 74%; (B) Conditions and reagents: (compound 32) NaNO3, H2SO4, 0 °C, 95%; (compound 33) H2, 10% Pd/C, EtOH, RT, 97%; (compound 34) 1. NaNO2; 2. NaN3, 75% AcOH, 0 °C, 66%; (compound 35) POCl3, DMF, 0 °C, 88%; (compound 36) 4-acetyl-pyridine, piperidine, MeOH, reflux, 39%; (compound 37) NaNO3, H2SO4, 0 °C, 62%; (compound 38) H2, 10% Pd/C, EtOH, RT, 99%; (compound 39) 1. NaNO2; 2. NaN3, 75% AcOH, 0 °C, 53% (compound 40) POCl3, DMF, 0 °C, 69%; (compound 41) 4-acetyl-pyridine, piperidine, MeOH, reflux, 39%.