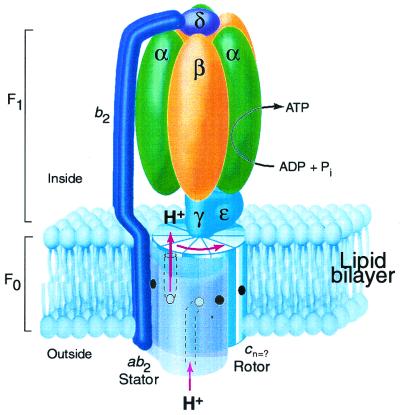
Figure 1.
Rotary model for ATP synthase based on the subunit composition of the E. coli enzyme (8). The F1 portion of the complex is bound at the cytoplasmic face of the membrane. The proton-motive force drives rotation of a ring composed of c subunits, the number (n) of which is uncertain. Protons enter the assembly through a periplasmic inlet channel and bind to the Asp61 carboxylate (open circle) of a subunit c at the a1b2 stator interface. The protonated binding site then moves from the stator interface into the lipid phase of the membrane, and after n steps the proton is released at an outlet channel on the F1-binding side of the membrane. The γ and ɛ subunits remain fixed to the top of a set of c subunits so that rotation of the c oligomer also drives rotation of subunit γ within the α3β3 hexamer of F1 to alternatively promote ADP + Pi binding and ATP product release in the β catalytic subunits. The b2 and δ subunits of the stator hold the α3β3 subunits in a fixed position as the γ subunit turns inside them to drive ATP synthesis. The coupled reaction catalyzed by the complex is reversible so that ATP hydrolysis will drive proton transport in the reverse of the direction shown. This figure is modified from ref. 8.