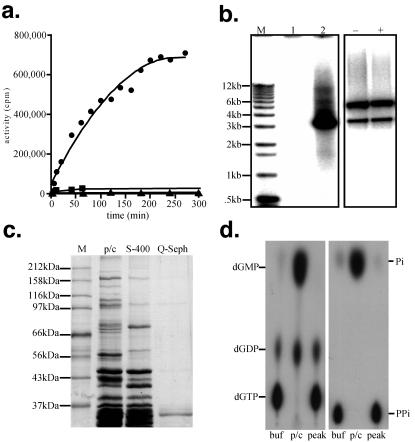
Figure 1.
Characteristics of DHBV core preparations (20). (a) Progress curve comparing the polymerase activity of cores purified by the newly developed method (●) versus those isolated by using a previous method (■), and preparations from uninfected animals (▴). (b) Agarose gel electrophoretic analysis of DNA polymerase reaction products after extended incubation time. End-labeled 1-kb ladder (GIBCO/BRL) (lane M), polymerase reaction products purified without (lane 1) and with (lane 2) protease pretreatment were subjected to gel electrophoresis and detected by autoradiography. The ability of cores to exclude exogenous enzymes from their interiors was assessed by incubating without (−) or with (+) 10 μg of DNase for 1 h after DNA labeling in a 1 h polymerase assay. (c) SDS/PAGE analysis of protein content of precolumn (p/c), Sephacryl S-400 gel filtration purified (S-400), and Q-Sepharose purified core preparations (Q-Seph) standardized by polymerase activity. The predominant band in the Q-Seph lane was verified to be the core protein by immunoreactivity (20). (d) Evaluation of dNTPase and PPiase contamination of purified cores. [α-32P]dGTP, which was most sensitive to degradation, or 32PPi were incubated in buffer (buf), precolumn cores (p/c), and peak Q-Sepharose purified cores at 37°C for 60 min. Breakdown products were separated by TLC, and visualized by autoradiography. Data partly adapted with permission from ref. 20.