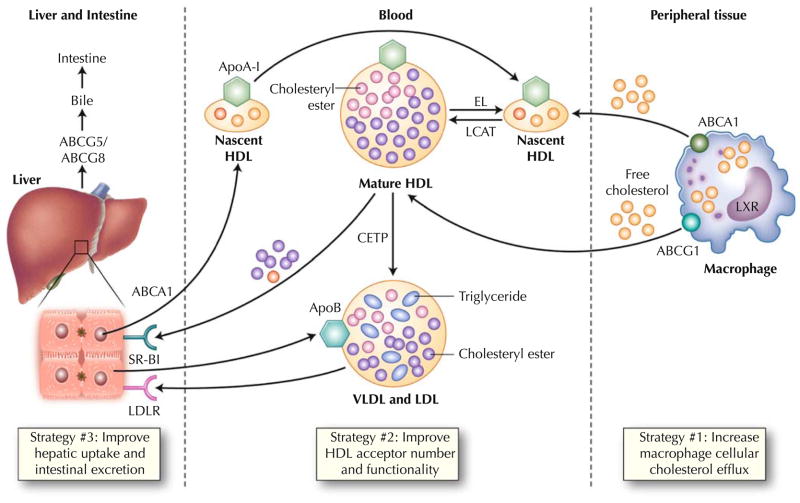
Fig. 1.
Physiology of macrophage reverse cholesterol transport. Both the liver and intestine synthesize apolipoprotein A-I (ApoA-I), which is secreted in lipid-poor form. These particles are lipidated with both phospholipids and free cholesterol via the hepatocyte ATP-binding cassette A1 (ABCA1) transporter to form nascent high-density lipoprotein (HDL). In peripheral tissues, these HDL particles obtain additional free cholesterol via the macrophage ABCA1 transporter. Lecithin cholesterol acyltransferase (LCAT) esterifies free cholesterol to cholesteryl esters, generating mature HDL. These larger HDL particles serve as additional acceptors of cholesterol efflux via the macrophage ATP-binding cassette G1 (ABCG1) pathway. Liver X receptor (LXR) regulates the expression of both ABCA1 and ABCG1 in the macrophage. HDL can be remodeled by lipases such as hepatic lipase and endothelial lipase (EL), which hydrolyze HDL triglyceride and phospholipid, respectively. Mature HDL can transport its cholesterol directly to the liver via the hepatic scavenger receptor class B type 1 (SR-B1) receptor. Alternatively, cholesteryl ester transfer protein (CETP) can mediate transfer of cholesteryl esters from HDL particles to apoB-containing lipoproteins with subsequent uptake in the liver via the low-density lipoprotein receptor (LDLR). This “indirect” pathway is thought to predominate in humans. Hepatic cholesterol is secreted into the bile via the ABCG5 and ABCG8 transporters. Some reenters the circulation via intestinal reabsorption, and the remainder is excreted into the feces. Recent evidence suggests that the intestine may be able to directly excrete cholesterol, bypassing the liver entirely. VLDL—very low density lipoprotein