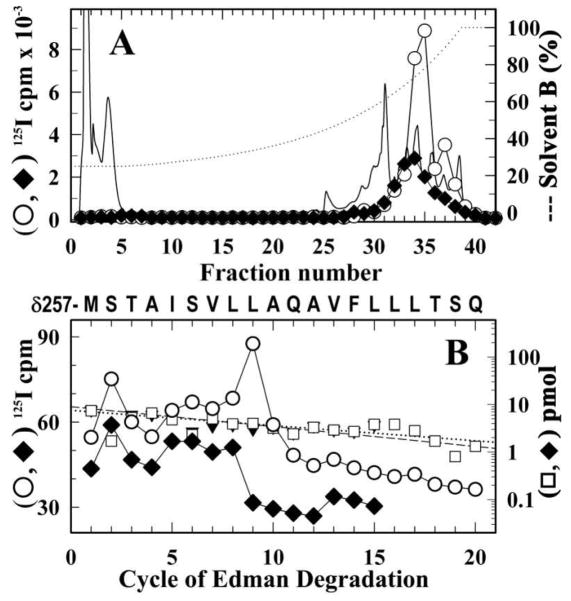
Figure 7. Identification of amino acids photolabeled by [125I]-SADU-3-72 in the δ M2 segment in the desensitized Torpedo nAChR.
nAChR-rich membranes were labeled with [125I]-SADU-3-72 in the presence of 400 μM Carb and in the absence (○, □) or presence 130 μM TCP (◆, ▼). As detailed in Experimental Procedures, the δ subunit was isolated from an 8% SDS-PAGE gel and digested ‘in-gel’ with V8 protease. The labeled fragment δV8-14K was isolated and further digested with trypsin. The fragment δT5K was then isolated from a small-pore Tricine SDS-PAGE gel. Shown are reversed-phase HPLC fractionation (A) and amino acid sequence analysis (B) of [125I]-SADU-3-72-labeled proteolytic fragments δT-5K (A). The elution of peptides during HPLC was monitored by absorbance at 210 nm (solid line) and 125I elution was quantified by γ counting of each fraction (○, ◆). B, 125I (○, ◆ ) and PTH-amino acids (□, ▼) released during amino acid sequence analysis of 125I peak (fractions 33–35; ○, 7,000 cpm; ◆, 3,000 cpm) from the HPLC purification of δT-5K. In each sample, the primary peptide detected began at δMet257 (□, I0 = 9 ± 1 pmol, R = 91%; ▼, I0 = 8 ± 1 pmol, R = 93%) and for the (◆) sample there were peaks of 125I cpm release in cycles 2 and 9 corresponding to labeling of δSer258 (3 cpm/pmol) and δLeu265 (5 cpm/pmol).