Abstract
Purpose
To investigate the effect of prostaglandin F2α (PGF2α), latanoprost, travoprost, bimatoprost, and tafluprost on human orbital preadipocyte differentiation and intracellular lipid storage, and to reveal the potential mechanisms by which topical prostaglandin analogs induce orbital fat volume reduction and cause deep superior sulcus syndrome.
Methods
Human orbital adipose precursors were treated in vitro for 24 h (day 1) with PGF2α, latanoprost, travoprost, bimatoprost, and tafluprost in their commercial formulations (1:100 dilution). Expressions of adipogenic transcription factor, peroxisome proliferator-activated receptor-gamma (PPARγ), and CCAAT-enhancer-binding protein α (C/EBPα) were determined by real-time reverse transcription-polymerase chain reaction (RT-PCR) at day 7. At 14 days, cells were stained with oil red O, intracellular lipid accumulation was evaluated by lipid absorbance, and adipocyte expression marker [Lipoprotein lipase (LPL)] was determined by real-time RT-PCR.
Results
Our results showed that PGF2α and topical prostaglandin analogs down-regulated the expression of PPARγ and C/EBPα, and inhibited accumulation of intra-cytoplasmic lipid droplets and expression of LPL compared with the untreated control. Comparison between the 4 drugs showed that latanoprost had the weakest antiadipogenic effect, and bimatoprost induced the most significant reduction of adipogenesis.
Conclusion
Latanoprost, travoprost, bimatoprost, and tafluprost inhibited human preadipocyte differentiation and intracellular lipid accumulation. Morphologic and metabolic changes in orbital adipocytes caused by PGF2α analogs are a possible pathophysiologic explanation of superior eyelid deepening in patients with glaucoma.
Introduction
The prostaglandins, lipid compounds derived enzymatically from fatty acids, are hormone-like substances that have numerous physiological functions in the human body.1 Prostaglandins ligate a sub-family of cell surface transmembrane receptors, G-protein-coupled receptors, and there are currently 10 known prostaglandin receptors on various cell types.2 The diversity of receptors means that prostaglandins act on the various cell cycle in many different cells and have a wide variety of actions including constriction or dilation in vascular smooth muscle cells, aggregation or disaggregation of platelets, decreasing intraocular pressure, regulation of inflammatory mediation, control of cell growth or differentiation, and lipolysis or lipogenesis of adipocytes.3
In ophthalmic applications, prostaglandin F2α (PGF2α) analogs reduce intraocular pressure by increasing the uveoscleral outflow and may also have some effect on the trabecular meshwork.3–7 Several commercially available topical medications have been developed, such as latanoprost, travoprost, bimatoprost, and the newly introduced tafluprost. These are synthetic PGF2α analogs and high-affinity agonists for the selective prostaglandin FP2α receptor.4–7 These agents have progressively become the first line topical treatments for ocular hypertension and glaucoma due to their efficacy, potency, and good patient compliance.
However, these drugs have several well-known side effects, most notably conjunctival hyperemia, ocular irritation, iris pigmentation, eyelid skin darkening, and eyelash hypertrichosis. Previous studies have proposed stimulation of melanogenesis in the skin or iris, disruption of the blood-aqueous barrier in pseudophakies, and the release of nitric oxide as possible mechanisms underlying the adverse effects of prostaglandin analogs.8–10 Recently, deepening of the upper eyelid sulcus accompanied by enophthalmos has been reported, not only for bimatoprost and travoprost, but also in some long-term users of latanoprost.11–20 Moreover, this new periorbital adverse effect has been noted only after use of topical prostaglandin analogs and has not been reported for other topical antiglaucoma drugs.
It is likely that these topical prostaglandin analogs have common pharmacological features, at least in part involving activation of the same receptor as PGF2α.
Considering these observations, we hypothesized that the action of PGF2α analogs on orbital adipocytes might be a possible mechanism for the deepening of the upper lid sulcus. We, therefore, investigated this possibility by comparing the effect of PGF2α analogs on in vitro adipose differentiation and adipogenesis using primary cultured human orbital adipose precursors.
Methods
Collection of human adipose tissue
The study design and protocols were approved by the institutional review board of Pusan National University Yangsan Hospital, and tissue was collected with informed consent. Orbital adipose tissue was obtained from 10 young (between 20 and 30 years old) and nonobese patients (body mass index <25 kg/m2) during elective orbital and eyelid reconstructive surgery. Patients with orbital tumors, history of using exogenous corticosteroids, or an underlying endocrine disease were excluded from the study.
Orbital preadiopcyte isolation and adipogenic differentiation
To isolate orbital preadipocytes, biopsied orbital adipose tissues were extensively washed with equal volumes of phosphate-buffered saline (PBS), minced with sterile scissors, and digested at 37°C for 45 min with 0.075% collagenase type I (Sigma). Enzyme activity was neutralized with Dulbecco's modified Eagle's medium (DMEM)/nutrient mixture F-12 (DMEM/F12; Gibco), containing 10% fetal bovine serum (FBS) and centrifuged (1,200 g, 10 min) to obtain a pellet. The pellet was filtered through a 100-μm nylon mesh to remove cellular debris and incubated overnight at 37°C/5% CO2 in control medium (DMEM/F12, 10% FBS, 100 unit/mL penicillin, and 100 μg/mL streptomycin). After incubation, the plates were extensively washed with PBS to remove residual nonadherent cells. The resulting cell population was maintained at 37°C/5% CO2 in control media. One week later, when a monolayer of adherent cells had reached confluence, cells were trypsinized (0.05% trypsin-EDTA; Sigma) and re-suspended in fresh media.
Adipogenic differentiation was induced by culturing tissue-mesenchymal stem cells (T-MSCs) for 2 weeks in adipogenic media (1 μM dexamethasone, 100 μg/mL 3-isobutyl-1 methylxanthine, 5 μg/mL insulin, and 60 μM indomethacine, 10% FBS in alpha-minimum essential medium [α-MEM]) and assessed by oil red O (Sigma) staining as an indicator of intracellular lipid accumulation. Before staining, the cells were fixed for 15 min at room temperature in 70% ethanol. Cells were then incubated in 2% oil red O reagent for 1 h at room temperature. Excess stain was removed by washing with 70% ethanol and distilled water to visualize lipid droplets.
To determine the extent of adipose conversion, we measured lipid absorbance of treated and untreated adipocytes, at 14 after adipose differentiation. After addition of 1 mL isopropyl alcohol to the stained culture disk, the extracted dye was immediately removed by gentle pipetting, and its absorbance was spectrophotometrically monitored at 510 nm.
PGF2α, latanoprost, travoprost, bimatoprost, and tafluprost treatment
We investigated the effects of PGF2α, latanoprost (Xalatan®; Pfizer), travoprost (Travatan®; Alcon), bimatoprost (Lumigan®; Allergan), and tafluprost (Taflotan®; Santen) in their commercial formulations on adipose differentiation and intracellular lipid storage. PGF2α was tested at a concentration of 10−4 M, corresponding to 35.5 μg/mL,9 which is comparable to the concentration of prostaglandin analogs in the 4 types of eye drop.
We examined each solution at the initial concentrations, diluted at 1:10, 1:100 and 1:1,000, after 24, 48, and 72 h of contact for MTT assay. Solutions tested at a 1:10 dilution appeared too toxic to be analyzed, even at 24 h. However, compounds tested at a 1:1,000 dilution did not change any marker with any drug (data not shown). We, therefore, focused our results on the 1:100 dilution with 24 h contact, which showed significant changes over time with the experiment.
Seven days after adipose differentiation with drug treatment (1:100 dilution and 24 h contact at day 1), adipogenic transcription factors were evaluated by real-time reverse transcription-polymerase chain reaction (RT-PCR). Intracellular fat accumulation of mature adipocyte was quantified by oil red-O staining absorbance, and adipocyte expressed marker at terminal differentiation was investigated by real-time RT-PCR, at 14 days after differentiation.
Real-time RT-PCR
Total RNA extraction
Total RNA was extracted from differentiated adipogenic cells at 7 and 14 days, using QIAzol™ Lysis Reagent (Qiagen). Briefly, samples were lysed in QIAzol lysis reagent, 1/5 volume of chloroform was added, and the mixture was shaken vigorously for 15 s and kept on ice for 5 min. The suspension was centrifuged at 12,000 rpm at 4°C for 15 min. The upper aqueous phase was transferred to a fresh tube, mixed with an equal volume of isopropanol, and kept on ice for 5 min. RNA was precipitated by centrifugation at 12,000 rpm at 4°C for 15 min. The RNA pellets were washed with 75% ethanol by vortexing and subsequent centrifugation at 12,000 rpm at 4°C for 5 min. The pellets were briefly dried and dissolved in diethypyrocarbonate-treated RNase-free solution. Purified RNA was incubated with DNase I (Invitrogen™) Solution for 15 min at 37°C, followed by 65 for 20 min, then placed on ice. Total RNA concentration was determined by measuring absorbance at 260 nm.
RT of RNA
Prepared RNA was reverse transcribed into complementary DNA (cDNA) in a 20 μL volume containing 5×RT Reaction buffer plus 0.005 M DTT, 1 mM each dNTP, 20 U RNase Inhibitor, 50 μM oligo(dT)17 primer, 2 μg total RNA, and 200 U of DiaStar™ RTase (SolGent). The reaction mixture was incubated at 50°C for 50 min, followed by 70°C for 10 min.
Real-time PCR
CCAAT-enhancer-binding protein α (C/EBPα) and peroxisome proliferator-activated receptor-gamma (PPARγ) were used as genetic markers of adipogenesis, and lipoprotein lipase (LPL) was assayed as terminal markers of adipocyte differentiation. Quantitative detection of C/EBPα, PPARγ, and LPL mRNA was performed by real-time PCR with the commercially available LightCycler FastStart DNA Master SYBR Green I (Roche) using the LightCycler instrument (Roche Molecular Systems), and all subsequent quantification steps were carried out according to the manufacturer's instructions.
The PCR primer pairs for cDNA amplification were as follows: human C/EBPα (sense) 5′- CCTTGTGCAATGTGAATGTGC-3′ and (antisense) 5′-CGGAGAGAGTCTCATTTTGGCAA-3′; human PPARγ (sense) 5′-TGAATGTGAAGCCCATTGAA-3′ and (antisense) 5′-CTGCAGTAGCTGCACGTGTT-3′; human LPL (sense) 5′-GAGATTTCTCTGTATGGCACC-3′ and (antisense) 5′-CTGCAAATGAGACACTTTCTC-3′; human GAPDH (sense) 5′-GGGGAGCCAAAAGGGTCATCATCT-3′ and (antisense) 5′- GAGGGGCCATCCACAGTCTTCT-3′. A typical 20 μL PCR reaction contained 1 μL of sample cDNA or a serial dilution of standard cDNA. PCR amplifications were performed in separate tubes for 50 cycles (10 s at 95°C; 5 s at 56°C; 13 s at 72°C) using PCR master mixtures specific for C/EBPα, PPARγ, LPL or the housekeeping gene GAPDH. The GAPDH reaction product served as a control for the PCR and as a reference for relative quantification of C/EBPα, PPARγ, and LPL mRNA.
The number of PCR cycles to reach the fluorescence threshold was defined as the cycle threshold (Ct). The Ct value for each sample was proportional to the log of the initial amount of input cDNA. By plotting the Ct value of an unknown sample on the standard curve, the amount of target sequence in the sample could be calculated. To normalize the C/EBPα, PPARγ, and LPL mRNA expression for differences in RNA input, RNA quality, and reverse transcriptase efficiency between samples, we also amplified the housekeeping gene GAPDH. From the standard curve, we derived the amounts of C/EBPα, PPARγ, LPL, and GAPDH. The ratio between the calculated amounts of PPARγ, C/EBPα, LPL, and GAPDH represented the normalized PPARγ (N PPARγ), C/EBPα (N C/EBPα), and LPL (N LPL) from separate experiments. The relative expression values of C/EBPα, PPARγ, and LPL of each of the groups are presented as the mean fold changes±standard deviation.
Statistical analysis
Statistical analyses were performed to evaluate differences in the expressions of PPARγ, C/EBPα, LPL, and lipid absorption among the groups. Kruskal–Wallis 1-way analysis of variance on ranks was performed to compare the relative expression values among groups, followed by Tukey's test. The Wilcoxon rank sum test was used to analyze the statistical significance between 2 groups. Statistical significance was determined at P values <0.05.
Results
Down-regulation of adipogenic transcription factors in cultured human orbital preadipocytes by PGF2α, latanoprost, travoprost, bimatoprost, and tafluprost
We compared the effect of PGF2α, latanoprost, travoprost, bimatoprost, and tafluprost on the differentiation of adipose precursors by measuring the expression of the following adipogenic transcription factors:
Peroxisome proliferator-activated receptor-gamma
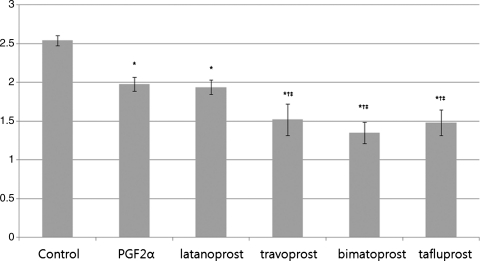
The mRNA level of PPARγ in cells treated with PGF2α, latanoprost, travoprost, bimatoprost, or tafluprost was significantly different from that of control cells (P=0.02). Comparison between PGF2α and PPARγ expression for latanoprost showed no significant difference in (P=0.56), but significantly reduced expression for travoprost (P<0.001), bimatoprost (P<0.001), and tafluprost (P<0.001). There was no significant difference between travoprost, bimatoprost, and tafluprost (P=0.09, Fig. 1).
FIG. 1.
Result of peroxisome proliferator-activated receptor-gamma (PPARγ) expression in cultured preadipocytes determined by real-time reverse transcription-polymerase chain reaction (RT-PCR), at 7 days after adipose differentiation. The values were calculated from separate experiments, and data were presented as the mean±standard deviations (SD). *Statistically significant difference compared with control; †Statistically significant difference compared with PGF2α; ‡Statistically significant difference compared with latanoprost. PGF2α, prostaglandin F2α.
CCAAT-enhancer-binding protein α
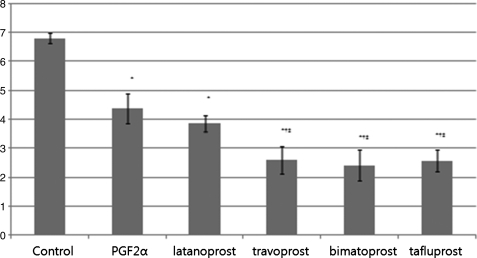
Expression of C/EBPα in cells treated with PGF2α, latanoprost, travoprost, bimatoprost, and tafluprost was significantly different from that of control cells (P=0.01). There was no significant difference between PGF2α and latanoprost (P=0.89), whereas travoprost (P=0.04), bimatoprost (P=0.04), and tafluprost (P=0.03) resulted in a significant reduction in C/EBPα expression compared with PGF2α-treated cells. C/EBPα expression in travoprost-treated cells was not significantly different from that of bimatoprost-treated (P=0.4) and tafluprost-treated group (P=0.8, Fig. 2).
FIG. 2.
Expression level of CCAAT-enhancer-binding protein α (C/EBPα) determined by real-time RT-PCR, at 7 days after adipose differentiation. Data were expressed as mean±SD. *Statistically significant difference compared with control; †Statistically significant difference compared with PGF2α.
Down-regulation of adipocyte expressed gene in cultured human orbital adipocyte by PGF2α, latanoprost, travoprost, bimatoprost, and tafluprost
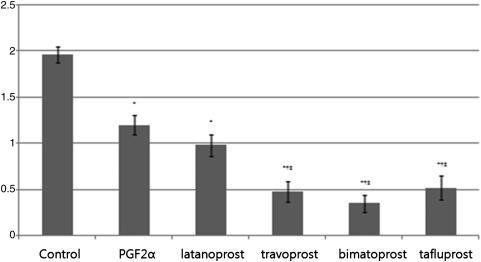
Adipocyte expressed gene LPL in cells treated with PGF2α, latanoprost, travoprost, bimatoprost, and tafluprost was significantly different from that of the control group (P=0.023). There was no significant difference between PGF2α and latanoprost (P=0.68) treated groups, while travoprost (P=0.03), bimatoprost (P=0.02), and tafluprost (P=0.03) resulted in a significant different in LPL expression compared with the PGF2α-treated group. Expression of LPL in travoprost-treated cells was not significantly different from that of the bimatoprost-treated (P=0.61) and tafluprost-treated group (P=0.92, Fig. 3).
FIG. 3.
Relative gene expressions of Lipoprotein lipase (LPL) at 14 days after adipose differentiation were assayed by real-time RT-PCR. The values were presented as the mean±SD. *Statistically significant difference compared with control; †Statistically significant difference compared with PGF2α.
The effect of PGF2α, latanoprost, travoprost, bimatoprost, and tafluprost on human orbital preadipocyte differentiation and intracellular lipid storage of mature adipocyte
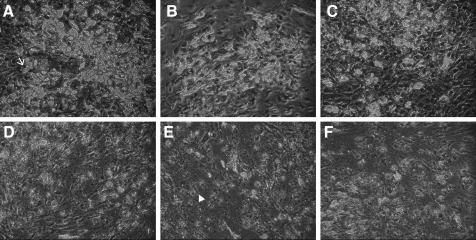
Orbital preadipocytes resembled fibroblasts but on differentiation the cells gradually changed to the distinctive morphology of mature adipocytes, which store lipid globules and are positively stained by oil Red-O, whereas undifferentiated cells retained their fibroblastic appearance throughout the duration of the experiments, and intracellular lipid granule was not observed (Fig. 4).
FIG. 4.
Phase-contrast microscopic photographs of adipocytes in 2 weeks after differentiation defined medium only (control) (A), PGF2α (B), latanoprost (C), travoprost (D), bimatoprost (E), and tafluprost (F). Differentiated cells showed marked lipid accumulation and round cell morphology (arrow), whereas undifferentiated cells retained their spindle-like and fibroblastic appearance throughout the duration of the experiment (arrow head, ×100).
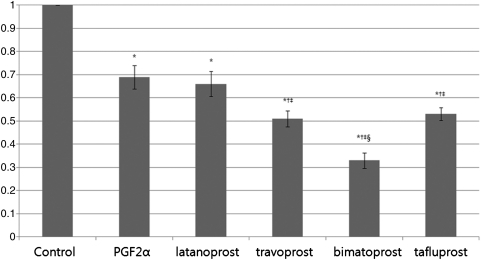
The absorption spectra of intracellular fat content in treated groups were significantly different from those of the control group. Latanoprost was not significantly different from PGF2α (P=0.67), while travoprost (P=0.04), bimatoprost (P<0.001), and tafluprost (P=0.001) were significantly different compared with PGF2α. The effect of travoprost was significantly different from that of bimatoprost (P=0.03), but showed no significant difference when compared with tafluprost (P=0.9, Fig. 5).
FIG. 5.
Quantification and comparison of adipose conversion determined by lipid absorbance of treated and untreated adipocyte, at 14 days after adipose differentiation. The values of experimental groups were compared with those of the control, which was normalized to 1.0. Result was represented as the mean±SD and calculated from separate experiments. *Statistically significant difference compared with control; †Statistically significant difference compared with PGF2α; ‡Statistically significant difference compared with latanoprost; §statistically significant difference compared with travoprost.
Discussion
The use of prostaglandin analogs in the medical therapy of glaucoma and ocular hypertensive patients has enormously grown, primarily because of their efficacy in reducing the intraocular pressure and the low rate and severity of adverse effects, especially systemic side effects. However, there are recent reports of deepening of the eyelid superior sulcus after long-term instillation of topical prostaglandin analogs that was recovered by discontinuation of the topical prostaglandin therapy,11–20 and some of these patients have shown enophthalmos combined with superior sulcus deepening.14–16,20 This recently recognized complication causes a disparity in the appearance of the eyelids and could be a reason for stopping glaucoma drug therapy.
Several possible mechanisms for these periorbital changes in users of prostaglandin analogs have been suggested. One proposed theory is that repeated stretching of the upper eyelid during topical drug instillation or mechanical rubbing may affect the Müller muscle and consequently lead to sulcus deepening.17,18 However, this is unlikely, as other topical solutions that are used more frequently are not known to cause this side effect. Another hypothesis is fatty degeneration combined with reduction in the collagen fibers of the levator complex, or Müller muscle fibrosis.12,21 According to this hypothesis, upper eyelid changes are permanent, and upper eyelid retraction or lid lag should also be observed. However, data in the literature and our clinical experience indicate that these changes are reversed after the patient stops using the drug, and upper eyelid retraction or lid lag were not observed in most patients with upper eyelid sulcus deepening.
It was reported that these patients show not only upper eyelid sulcus deepening, but also a reduced infraorbital fat pad, and combined enophthalmos.14,15 Periorbital fat atrophy was also demonstrated on MRI scaning.16 It was, therefore, inferred that the common pathophysiologic mechanism of these periocular changes was atrophy of orbital fat. Park et al. also revealed that the mean adipocyte densities of prostaglandin-treated eyes were statistically significantly higher than those of untreated eyes.20 They suggested that fat atrophy could be a major mechanism of upper eyelid sulcus deepening in patients using topical prostaglandin analogs,20 as orbital volume reduction in anophthalmos is the main cause of deepening of the upper eyelid sulcus.22
Orbital fat tissue represents a highly specialized adipose tissue depot that occupies the space behind the eyeball.23 This specialized fat tissue fills most of the orbital cavity and supports the globe, extraocular muscles, nerves, levator, eyelid, and vessels, thus alteration of the orbital fat tissue might be related to changes in orbital volume. Orbital fat tissue is biologically distinct from the omental and subcutaneous fat tissue, not only in morphological phenotypic features, but also at a molecular level with respect to expression of distinct surface receptors, cellular proteins, and responses to cytokines or hormones.23,24 Moreover, orbital adipocytes are significantly smaller and express lower levels of adipocyte differentiation markers compared with cells of the omental and subcutaneous fat tissue.24 Hence, inhibition of preadipocyte differentiation or reduction of fat accumulation in adipocytes caused by hormones, drugs, or prostaglandins might be linked to orbital volume reduction, and volume deficits of the orbit may, in turn, lead to deep superior sulcus syndrome and enophthalmos.
Several studies have reported the effects of prostaglandins in adipocytes: prostaglandin EP3 agonist inhibits lipolysis and J2 agonist activates adipogenesis, whereas adipocytes cultivated in the presence of PGF2α agonists remain fibroblastic, typical of undifferentiated adipocytes,25–28 and demonstrate inhibition of glycerol-3-phosphate dehydrogenase activity, an indicator of fat biosynthesis and triglyceride accumulation in the cell cytoplasm, and decreased expression of adipocyte markers.25 The proposed cellular mechanism is as follows: PGF2α binds to the cell surface FP receptor and activates mitogen-activated protein kinase, which exerts an antiadipogenic effect through inhibition of a nuclear hormone receptor, PPARγ. As a result, differentiation of orbital fibroblasts into adipocytes is blocked. These effects are also observed in mature adipocytes.29
n our study, PGF2α and prostaglandin analogs inhibited preadipocyte differentiation markers and reduced fat accumulation at the mature stage compared with the control group. In addition, we observed that greater suppression of preadipocyte differentiation tended to be linked to more significant reduction of adipose differentiation terminal marker and fat accumulation in adipocytes. Comparison between the drugs showed that bimatoprost had the most significant inhibitory effect on adipose differentiation and intracellular lipid accumulation, while latanoprost seemed to have the weakest antiadipogenic effect. This is consistent with previous clinical reports and histology studies; upper eyelid sulcus deepening was most frequently reported in bimatoprost users,12–17 and histopathologic study showed that bimatoprost- and travoprost-treated groups had significantly different cell density compared with latanoprost-treated and untreated groups.20 Although deepening of superior sulcus has not been reported in a recently introduced drug, tafluprost, our results suggested that antiadipogenesis could be occurred in tafluprost users.
Several studies have shown that PGF2α analogs exhibit different agonist activities in experimental and clinical applications; this has been attributed to structural differences between the PGF2α analogs, differences in FP receptor affinity, or minor effects on other prostaglandin receptors.30,31 Further studies are obviously needed to determine the mechanisms underlying the specific molecular affinity of different PGF2α analogs for adipocytes.
In conclusion, we suggest that topical prostaglandin analogs affect the prostaglandin FP2α receptors of orbital preadipocytes and inhibit the differentiation of adipose precursors and lipid content of adipocytes. Therefore, these antiadipogenic effect of PGF2α analogs would be involved in orbital fat atrophy or adipocyte degradation.
This inhibitory effect on orbital adipose precursors and adipogenesis could be an important pathophysiologic mechanism of upper eyelid sulcus deepening in topical prostaglandin analog users.
Obviously, since these results were obtained in vitro, they cannot be fully extrapolated to in vivo conditions, but our experimental results are in good agreement with data from the literature assessing superior sulcus deepening. This preliminary in vitro study of 4 major prostaglandin analogs suggests that inhibition of preadipocyte differentiation and fat metabolism by PGF2α analogs is associated with morphologic and metabolic changes in orbital adipocytes, and indicates a possible pathophysiologic mechanism of superior eyelid deepening.
Acknowledgments
This study was supported by Medical Research Institute Grant (2011-20), Pusan National University Hospital, Busan, Korea.
The funding organization had no role in the design or conduct of this research.
Author Disclosure Statement
No conflicting relationship exists for any author.
References
- 1.Tsuboi K. Sugimoto Y. Ichikawa A. Prostanoid receptor subtypes. Prostaglandins Other Lipid Mediat. 2002;68–69:535–556. doi: 10.1016/s0090-6980(02)00054-0. [DOI] [PubMed] [Google Scholar]
- 2.Hata A.N. Breyer R.M. Pharmacology and signaling of prostaglandin receptors: multiple roles in inflammation and immune modulation. Pharmacol. Ther. 2004;103:147–166. doi: 10.1016/j.pharmthera.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Ishida N. Odani-Kawabata N. Shimazaki A. Hara H. Prostanoids in the therapy of glaucoma. Cardiovasc. Drug Rev. 2006;24:1–10. doi: 10.1111/j.1527-3466.2006.00001.x. [DOI] [PubMed] [Google Scholar]
- 4.Hylton C. Robin A.L. Update on prostaglandin analogs. Curr. Opin. Ophthalmol. 2003;14:65–69. doi: 10.1097/00055735-200304000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Camras C.B. United States Latanoprost Study Group. Comparison of latanoprost and timolol in patients with ocular hypertension and glaucoma: a six-month, masked, multicenter trial in the United States. Ophthalmology. 1996;103:138–147. doi: 10.1016/s0161-6420(96)30749-5. [DOI] [PubMed] [Google Scholar]
- 6.Parrish R.K. Palmberg P. Sheu W.P. XLT Study Group. A comparison of latanoprost, bimatoprost, and travoprost in patients with elevated intraocular pressure: a 12-week, randomized, masked-evaluator multicenter study. Am. J. Ophthalmol. 2003;135:688–703. doi: 10.1016/s0002-9394(03)00098-9. [DOI] [PubMed] [Google Scholar]
- 7.Sagara T. Gaton D.D. Lindsey J.D., et al. Topical prostaglandin F2 treatment reduces collagen types I, III, and IV in the monkey uveoscleral outflow pathway. Arch. Ophthalmol. 1999;117:794–801. doi: 10.1001/archopht.117.6.794. [DOI] [PubMed] [Google Scholar]
- 8.Vogel R. Strahlman E. Rittenhouse K.D. Adverse events associated with commonly used glaucoma drugs. Int. Ophthalmol. Clin. 1999;39:107–124. doi: 10.1097/00004397-199903920-00009. [DOI] [PubMed] [Google Scholar]
- 9.Guenoun J.M. Baudouin C. Rat P., et al. In vitro comparison of cytoprotective and antioxidative effects of latanoprost, travoprost, and bimatoprost on conjunctiva-derived epithelial cells. Invest. Ophthalmol. Vis. Sci. 2005;46:4594–4599. doi: 10.1167/iovs.05-0776. [DOI] [PubMed] [Google Scholar]
- 10.Holló G. The side effects of the prostaglandin analogues. Expert Opin. Drug Saf. 2007;6:45–52. doi: 10.1517/14740338.6.1.45. [DOI] [PubMed] [Google Scholar]
- 11.Schnober D. Hofmann G. Maier H., et al. Diurnal IOP-lowering efficacy and safety of travoprost 0.004% compared with tafluprost 0.0015% in patients with primary open-angle glaucoma or ocular hypertension. Clin. Ophthalmol. 2010;4:1459–1463. doi: 10.2147/OPTH.S13720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peplinski L.S. Albiani Smith K. Deepening of lid sulcus from topical bimatoprost therapy. Optom. Vis. Sci. 2004;81:574–577. doi: 10.1097/01.opx.0000141791.16683.4a. [DOI] [PubMed] [Google Scholar]
- 13.Lee J.W. Kim D.Y. Lee Y.K. Two cases of deepening of the upper lid sulcus from topical bimatoprost therapy. J. Korean Ophthalmol. Soc. 2007;48:332–336. [Google Scholar]
- 14.Filippopoulos T. Paula J.S. Torun N., et al. Periorbital changes associated with topical bimatoprost. Ophthal. Plast. Reconstr. Surg. 2008;24:302–307. doi: 10.1097/IOP.0b013e31817d81df. [DOI] [PubMed] [Google Scholar]
- 15.Tappeiner C. Perren B. Iliev M.E., et al. Orbital fat atrophy in glaucoma patients treated with topical bimatoprost can bimatoprost cause enophthalmos? Klin. Monbl. Augenheilkd. 2008;225:443–445. doi: 10.1055/s-2008-1027362. [DOI] [PubMed] [Google Scholar]
- 16.Jayaprakasam A. Ghazi-Nouri S. Periorbital fat atrophy—an unfamiliar side effect of prostaglandin analogues. Orbit. 2010;29:357–359. doi: 10.3109/01676830.2010.527028. [DOI] [PubMed] [Google Scholar]
- 17.Yam J.C. Yuen N.S. Chan C.W. Bilateral deepening of upper lid sulcus from topical bimatoprost therapy. J. Ocul. Pharmacol. Ther. 2009;25:471–472. doi: 10.1089/jop.2009.0019. [DOI] [PubMed] [Google Scholar]
- 18.Yang H.K. Park K.H. Kim T.W. Kim D.M. Deepening of eyelid superior sulcus during topical travoprost treatment. Jpn. J. Ophthalmol. 2009;53:176–179. doi: 10.1007/s10384-008-0623-x. [DOI] [PubMed] [Google Scholar]
- 19.Casson R.J. Selva D. Lash ptosis caused by latanoprost. Am. J. Ophthalmol. 2005;139:932–933. doi: 10.1016/j.ajo.2004.10.062. [DOI] [PubMed] [Google Scholar]
- 20.Park J. Cho H.K. Moon J.I. Changes to upper eyelid orbital fat from use of topical bimatoprost, travoprost, and latanoprost. Jpn. J. Ophthalmol. 2011;55:22–27. doi: 10.1007/s10384-010-0904-z. [DOI] [PubMed] [Google Scholar]
- 21.Yoshida M. Sagawa N. Itoh H., et al. Prostaglandin F (2alpha), cytokines and cyclic mechanical stretch augment matrix metalloproteinase-1 secretion from cultured human uterine cervical fibroblast cells. Mol. Hum. Reprod. 2002;8:681–687. doi: 10.1093/molehr/8.7.681. [DOI] [PubMed] [Google Scholar]
- 22.Hardy T.G. Joshi N. Kelly M.H. Orbital volume augmentation with autologous micro-fat grafts. Ophthal. Plast. Reconstr. Surg. 2007;23:445–449. doi: 10.1097/IOP.0b013e31815928f8. [DOI] [PubMed] [Google Scholar]
- 23.Wolfram-Gabel R. Kahn J.L. Adipose body of the orbit. Clin. Anat. 2002;15:186–192. doi: 10.1002/ca.10011. [DOI] [PubMed] [Google Scholar]
- 24.Bujalska I.J. Durrani O.M. Abbott J., et al. Characterisation of 11beta-hydroxysteroid dehydrogenase 1 in human orbital adipose tissue: a comparison with subcutaneous and omental fat. J. Endocrinol. 2007;192:279–288. doi: 10.1677/JOE-06-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Serrero G. Lepak N.M. Prostaglandin F2alpha receptor (FP receptor) agonists are potent adipose differentiation inhibitors for primary culture of adipocyte precursors in defined medium. Biochem. Biophys. Res. Commun. 1997;233:200–202. doi: 10.1006/bbrc.1997.6433. [DOI] [PubMed] [Google Scholar]
- 26.Reginato M.J. Krakow S.L. Bailey S.T. Lazar M.A. Prostaglandins promote and block adipogenesis through opposing effects on peroxisome proliferator-activated receptor gamma. J. Biol. Chem. 1998;273:1855–1858. doi: 10.1074/jbc.273.4.1855. [DOI] [PubMed] [Google Scholar]
- 27.Ajjan R.A. Weetman A.P. New understanding of the role of cytokines in the pathogenesis of Graves' ophthalmopathy. J. Endocrinol. Investig. 2004;27:237–245. doi: 10.1007/BF03345272. [DOI] [PubMed] [Google Scholar]
- 28.Fujimori K. Ueno T. Nagata N., et al. Suppression of adipocyte differentiation by aldo-keto reductase 1B3 acting as prostaglandin F2alpha synthase. J. Biol. Chem. 2010;285:8880–8886. doi: 10.1074/jbc.M109.077164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Serrero G. Lepak N. Endocrine and paracrine negative regulators of adipose differentiation. Int. J. Obes. Relat. Metab. Disord. 1996;20:S58–S64. [PubMed] [Google Scholar]
- 30.Sharif N.A. Kelly C.R. Crider J.Y., et al. Ocular hypotensive FP prostaglandin (PG) analogs: PG receptor subtype binding affinities and selectivities, and agonist potencies at FP and other PG receptors in cultured cells. J. Ocul. Pharmacol. Ther. 2003;19:501–515. doi: 10.1089/108076803322660422. [DOI] [PubMed] [Google Scholar]
- 31.Sharif N.A. Crider J.Y. Husain S., et al. Human ciliary muscle cell responses to FP-class prostaglandin analogs: phosphoinositide hydrolysis, intracellular Ca2+ mobilization and MAP kinase activation. J. Ocul. Pharmacol. Ther. 2003;19:437–455. doi: 10.1089/108076803322473006. [DOI] [PubMed] [Google Scholar]