Abstract
Effects of egg york containing IgY specific for Helicobacter pylori on the bacterial growth and intragastric infection were investigated in comparison with a proton-pump inhibitor pantoprazole. For in vitro anti-bacterial activity test, H. pylori (1×108 CFU/mL) was incubated with a serially diluted IgY for 3 days. As a result, IgY fully inhibited the bacterial growth at 16 mg/mL, which was determined to a minimal inhibitory concentration. In vivo elimination study, male C57BL/6 mice were infected with the bacteria by intragastric inoculation (1×108 CFU/mouse) 3 times at 2-day intervals, and 2 weeks later, orally treated twice a day with 50, 100, 200 or 500 mg/kg IgY for 18 days. After the final administration, biopsy sample of the gastric mucosa was assayed for the bacterial identification via urease, oxidase, catalase, nitrate reduction and H2S tests in addition to microscopic examination for mucosal inflammation. In CLO kit test, 75, 50, 12.5 and 12.5% of the animals revealed positive reaction following treatment with 50, 100, 200 and 500 mg/kg IgY, respectively, resulting in a superior efficacy at 200 mg/kg than 30 mg/kg pantoprazole that displayed 75% elimination. The CLO test results were confirmed by bacterial identification. Microscopic examination revealed that H. pylori infection caused severe gastric mucosal inflammation, which were not observed in the CLO-negative mice following treatment with IgY or pantoprazole. Taken together, IgY inhibited the growth of H. pylori, and improved gastritis and villi injuries by eliminating the bacteria from the stomach. The results indicate that IgY could be a good candidate overcoming tolerance of antibiotics for the treatment of H. pylori-mediated gastric ulcers.
Keywords: Helicobacter pylori, immunoglobulin Y (IgY), minimal inhibitory concentration (MIC), CLO test, gastric inflammation
Peptic ulcers are injury of the mucosal area immersed in gastric acid and pepsin, wherein the area is covered normally with mucin secreted from mucus cells [1,2]. So, erosions and ulcers are caused by various factors including gastric oversecretion and retention, weakening and depleting agents of mucin layer, blood flow disturbances, and mucosal injury and inflammation [1-4]. The ulcer-inducing agents include non-steroidal anti-inflammatory drugs (NSAIDs) that block production prostaglandins (PGs), leading to mucin depletion and blood flow disturbances [5-9], alcohols [7,8,10], stresses [4,7,8], gastric oversecretion and retention [7,8], gastric hypermotility and acetic acid accumulation [8,11-14] and bacterial (Helicobacter pylori) infection [1,2,15-17].
It is well known that H. pylori, closely associated with peptic ulcers, chronic active gastritis and gastric malignancies, is a key factor for recurrence and aggravation of ulcers as well as development to gastric malignancies [18-20]. It is believed that H. pylori aggravates ulcers by continuously stimulating gastric secretion [21,22]. Thus, elimination of H. pylori is a key point for ulcer treatment in adults exhibiting a high incidence [1,2,15,17,23,24]. For the therapy of H. pylori-mediated gastritis and ulcers, triple therapies (omeprazole+clerithromycin+amoxicillin or omeprazole+clerithromycin+metronidazole) composed of proton-pump inhibitors (pantoprazole, omeprazole, lansoprazole, etc) and antibiotics (clarithromycin, metronidazole, amoxicillin, etc) have been recommended [2]. However, antibiotics used for triple therapy are becoming ineffective due to a rapidly-increasing tolerance of the bacteria [25].
In the present study, in vitro anti-bacterial activity and in vivo bacteria-eliminating efficacy of IgY specific for H. pylori obtained by immunization to hens were investigated in comparison with a proton-pump inhibitor pantoprazole. It has been reported that proton-pump inhibitors such as pantoprazole inhibit gastric secretion [26,27], protect parietal cells [28-30], and suppress growth of H. pylori [31-33].
Materials and Methods
Materials
Ig-Guard Helico®, egg york containing anti-H. pylori IgY (9.5 mg/g), obtained from immunized hens, were from ADbiotech Co., Ltd. (Chuncheon, Korea). In brief, H. pylori (KCTC12083) was cultured in Brucella agar containing 5% fetal bovine serum (FBS) at a 10% CO2 incubator (37℃), further in Brucella broth, and inactivated in 0.3% formalin for 12 hours. The inactivated antigen was centrifuged at 9,000 rpm for 15 min (4), and the pellet was adjusted to optical density (OD) of 0.5 at 410 nm. After mixing the antigen with ISA 70 adjuvant (3:7), the vaccine sample (3 mL) was intramuscularly inoculated to 22-week-old Hy-Line brown hens 3 times at 3-week intervals followed by boosting vaccination 35 days after the final inoculation. IgY-containing egg york was stored at -70℃ until use.
H. pylori culture and identification
H. pylori (ATCC49503) was obtained from American Type Culture Collection (Manassas, VA, USA), and cultured on brain heart infusion (BHI) broth in an anaerobic chamber with 10% CO2, 5% O2 and 85% N2 at 37℃ with enough humidity.
In vitro anti-bacterial activity
The inhibitory capacity of IgY against the growth of H. pylori was assessed using agar-dilution method [32]. In brief, serially diluted IgY was added to Mueller Hinton agar containing 10% FBS, and H. pylori was inoculated on the agar. After 3-day incubation at 37℃, the IgY concentration fully inhibiting the bacterial growth was determined to be minimal inhibitory concentration (MIC).
Animals and treatment
Male C57BL/6 mice (body weights 20-24 g) were procured from Daehan Biolink (Eumseong, Korea), and housed in a room with constant environmental conditions (22±2℃; 40-70% relative humidity; 12-hour light-dark cycle; 150-300 lux brightness). Pellet feed and purified water were available ad libitum. All the animal experiments were conducted according to the Standard Operation Procedures (SOP), and approved by the Institutional Animal Care and Use Committee of Chungbuk National University, Korea. For biosafety the investigators were fully protected with sterilized clothes, masks and gloves on SOP.
H. pylori infection and treatment
After 24-hour fasting, the mice (n=10/group) were orally inoculated with H. pylori (1×108 CFU/1 mL/mouse) 3 times at 2-day intervals [17,24]. Fourteen days later, a part of the animals (2 mice/group) were sacrificed to confirm infection using CLO Helicobacter-detection kits (Asan Pharm Co., Ltd., Seoul, Korea). The mice were orally treated with IgY (50, 100, 200 or 500 mg/kg) or pantoprazole (30 mg/kg) twice a day for 18 days.
Biopsy and bacterial identification
One day after the final administration, the mice were sacrificed and their gastric mucosa was biopsied for the detection of H. pylori. The biopsy samples (3×3 cm) from gastric pylorus were minced and applied to CLO kits and incubated at 35℃ for 24 hours to examine urease activity. The reaction (color change) was determined as negative for bright yellow, false (partially) positive for thick yellow, or positive for thick (dark) red.
Additional biopsy samples were obtained with a culture swab and inoculated to a medium containing 20% glycerol for identification of H. pylori as follows:
Oxidase: A colony was collected with a platinum-loop, and moved to a filter paper. After dropping p-phenylenediamine dihydrochloride solution, blue change following indolphenol blue production was determined as positive reaction.
Catalase: The colony grown in selective medium was placed on a slide, and bubbling following dropping 3% H2O2 was determined as positive reaction.
Nitrate reduction: Cultured colony was inoculated to nitrate broth, and incubated at 35℃ for 48 hours. Reddish purple change by adding α-naphthylamine and sulfanilic acid was determined as positive reaction.
H2S formation: The central portion of a colony was collected with a platinum-loop, and spread on the slant Triple sugar iron (TSI) medium. Black change following incubation at 37℃ for 24 hours with the lead open was determined as positive reaction producing H2S.
Histopathological examination
Stomach was removed and fixed in neutral formalin solution. Paraffin-embedded tissue slides were stained with hematoxylin-eoson, and examined under a light microscope for the inflammatory lesions.
Statistical analysis
Data were expressed as the mean±SEM. Statistical analysis was performed using an analysis of variance (ANOVA) with the aid of SPSS for Windows v.10.0 (Chicago, Illinois, USA). A P value <0.05 was considered statistically significant.
Results
Anti-bacterial activity
In vitro anti-bacterial test, 16 mg/mL and higher concentrations of IgY completely inhibited the growth of H. pylori following 3-day incubation of the bacteria (1×108 CFU/mL) with a serially diluted IgY (Table 1). Therefore, MIC of IgY was determined to be 16 mg/mL, which was much higher than MIC of pantoprazole (2.2 mg/mL).
Table 1.
Minimum inhibitory concentration (MIC) of egg york (IgY) and pantoprazole
CLO kit test
Repeated intragastric inoculation (1×108 CFU, 3 times) of H. pylori to C57BL/6 mice revealed positive reaction (red color) in CLO test 14 days after the final inoculation, indicative of the bacterial infection in the stomach.
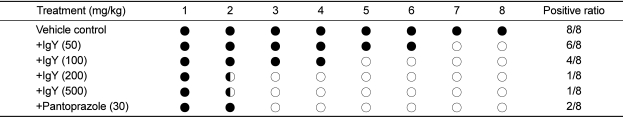
The mice orally treated with 50, 100, 200 or 500 mg/kg IgY twice a day for 18 days displayed positive reaction in 75% (6/8), 50% (4/8), 12.5% (1/8) and 12.5% (1/8) mice, respectively, in comparison with 100% (8/8) positivity at control (vehicle) group (Table 2). Although there was one case each in 200 and 500 mg/kg IgY group, no bacteria was detected in bacterial identification study. By comparison, 25% (2/8) positive reaction was achieved following treatment with pantoprazole (30 mg/kg).
Table 2.
Reactivity in CLO test on the gastric mucosa of mice infected with H. pylori followed by treatment with egg york IgY or pantoprazole
○, negative; ◐, partially positive; ●, positive.
Culture of gastric mucosa
From the culture of gastric mucosal specimen to confirm the presence of H. pylori, bacteria grew in all the samples from mice (100%) treated with vehicle (Table 3). However, IgY treatment decreased the detection ratio in a dose-dependent manner, leading to 70, 50, 12.5, and 12.5% at 50, 100, 200 and 500 mg/kg, respectively. Notably, there was no bacterial growth from the specimens that displayed partially positive reaction in CLO test. By comparison, pantoprazole (30 mg/kg) also reduced the detection ratio of bacterial growth to 25%.
Table 3.
Identification of H. pylori through culture of gastric mucosa from mice infected with H. pylori followed by treatment with egg york (IgY) or pantoprazole
-, negative; +, positive.
H. pylori identification
The colonies grown from the gastric specimen were identified whether they are H. pylori. In urease test, all the colonies were identified as H. pylori, as also confirmed in CLO test. However, all the samples from mice including normal and vehicle groups exhibited positive reactivity to oxidase and catalase, regardless of treatment with IgY or pantoprazole, which might be due to the enzymes from mucosal tissue or microorganisms other than infected H. pylori. On the contrary, nitrate reduction and H2S formation, negative markers of H. pylori, were negative in all the samples including normal, vehicle and treated groups, implying that there were no nitrate-reducing and H2S-producing microorganisms or tissue enzymes and other agents.
Histopathological findings
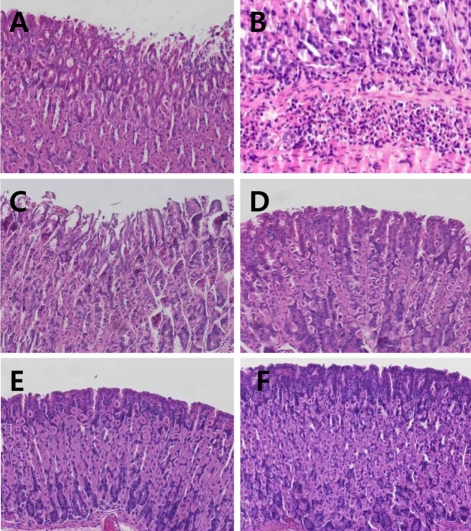
H. pylori infection led to degeneration and slough of gastric villi with severe submocosal infiltration of inflammatory cells (Figures 1A and 1B). IgY inhibited the inflammatory lesions, recovering to normal features with high doses (100-500 mg/kg), although light detachment of villi was observed at a low dose (50 mg/kg). Pantoprazole (30 mg/kg) also markedly improved the villi lesions. However, the histopathological features were in parallel with the presence of H. pylori as confirmed in CLO test and identification study.
Figure 1.
Representative microscopic findings of the gastric mucosa of mice infected with H. pylori followed by treatment with IgY (50-200 mg/kg) or pantoprazole (30 mg/kg). Note the degeneration and sloughing of villi (A) and submocosal inflammatory cell infiltration (B) in vehicle group, in comparison with light sloughing of villi at a low (50 mg/kg) dose of IgY (C) and near-normal features at high doses of IgY (D, 100 mg/kg; E, 200 mg/kg) or pantoprazole (F, 30 mg/kg).
Discussion
Repeated (3 times) intragastric inoculation of H. pylori (ATCC49503, 1×108 CFU/mouse) to male C57BL/6 mice revealed strong positive reaction in CLO test in 14 days, confirming good settlement of the bacteria. Interestingly, some normal animals without exposure to H. pylori displayed a weak (false or partially positive) reactivity, which may be due to the cross-reaction of other urease-producing bacteria such as Klebsiella and Proteus [34].
However, in comparison with the weak reactivity to ureases from Klebsiella and Proteus that are cytosolic proteins, CLO test detecting extracellular urease from Helicobacter is very strong so that the efficacy of the test is believed to be high. Notably, oxidase and catalase, positive identification markers of H. pylori, was displayed positive in normal animals too. Such a phenomenon might be due to the enzymes from tissues and/or other microorganisms. In contrast, nitrate reduction H2S formation, negative identification markers of H. pylori, was also negative in normal mice, indicating that there were no nitrate-reducing and H2S-producing microorganisms or tissue enzymes in the stomach. Such results point out that oxidase, catalase, nitrate reduction and H2S formation, besides urease, are not suitable markers for detection of H. pylori from biopsy specimens or in tissues. To overcome such a limitation of identification methods, we additionally examined the histopathological findings for the evaluation of H. pylori-induced inflammation and mucosal injuries including degeneration and sloughing of villi.
In vitro culture study, IgY exerted a relatively weak anti-H. pylori activity, with 1 mg/mL of MIC. The activity of IgY in our study was similar to that of H. pylori-specific IgY-Hp that inhibited bacterial growth and urease activity by 40 and 24.6% at 1 mg/mL, and 88 and 84.5% at 10 mg/mL, respectively [35]. In another study, H. pylori urease-specific IgY-HpU also inhibited urease activity by 29.9 and 81.7% at 1 and 10 mg/mL, respectively [36], leading to similar efficacies to IgY in the present study. On the other hand, IgY-HpUc specific for UreC subunit of urease exerted higher inhibitory effects on the growth and urease activity of H. pylori [37].
Treatment with IgY for 18 days eliminated H. pylori from the stomach of mice in a dose-dependent manner, reaching 87.5% at 200 or 500 mg/kg. Such anti-bacterial and anti-inflammatory effects of IgY seem to be higher than the efficacy of IgY-Hp that exhibited 50-60% anti-inflammatory activity at 180 mg/kg in a gerbil model [35]. Furthermore, the efficacy of IgY was superior to the H. pylori-elimination ratio (70%) of IgY-Hp58 specific for 58-kDa H. pylori (Hp) antigen [38]. On the other hand, it was reported that urease-specific IgY significantly attenuated gastric inflammation of gerbils fed at a concentration of 25 mg/g diet [39]. In the present study, H. pylori infection caused extensive degeneration and detachment of villi, accompanying severe infiltration of inflammatory cells. However, such pathological changes were remarkably recovered following eradication of bacteria by treatment of IgY or pantoprazole. It is of interest to note that bacterial identification was impossible in the animals showed partially-positive reaction in CLO test, which might be due to the urease released from the bodies or debris of dead H. pylori.
In previous studies, in vivo H. pylori-eliminating capacity of IgY specific for urease or subunit of urease has been demonstrated [37,40-42]. However, since the titer of such antibodies tended to rapidly decrease at low pH, researches has focused on their efficacy in the stomach [40,43]. Indeed, urease-specific IgY did not completely elliminated H. pylori from human patients, even though it markedly reduced expiratory urea concentration by alleviating gastritis and inhibiting urease activity [43,44]. Thus, human trial for our IgY is necessary to assess its clinical efficacy. Furthermore, since synergistic effects in anti-bacterial and anti-inflammatory activities between IgY and lansoprazole or famotidine were achieved [39,44,45], combinational therapy of IgY and traditional drugs is also recommended.
In the present study, it was found that IgY inhibit the growth of H. pylori with 16 mg/mL of MIC, and improve gastritis and villi injuries by eliminating the bacteria from the stomach of mice. The results indicate that IgY could be a good candidate overcoming tolerance of antibiotics for the treatment of H. pylori-mediated gastric ulcers.
Acknowledgments
This work was supported in part by ADbiotech Co. and Priority Research Centers Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010-0029709).
References
- 1.Wallace JL, Granger DN. The cellular and molecular basis of gastric mucosal defense. FASEB J. 1996;10(7):731–740. doi: 10.1096/fasebj.10.7.8635690. [DOI] [PubMed] [Google Scholar]
- 2.Neal MJ. Medical Pharmacology at a Glance. 3rd ed. London: Blackwell Publishing Inc; 2003. pp. 30–31. [Google Scholar]
- 3.Isobe H, Okajima K, Harada N, Liu W, Okabe H. Activated protein C reduces stress-induced gastric mucosal injury in rats by inhibiting the endothelial cell injury. J Thromb Haemost. 2004;2(2):313–320. doi: 10.1046/j.1538-7933.2003.00574.x. [DOI] [PubMed] [Google Scholar]
- 4.Byun SK, Lee YE, Shin SH, Jang JY, Choi BI, Park DS, Jeon JH, Nahm SS, Hwang SY, Kim YB. The role of corticosteroids in stress-induced gastric ulceration in rats. Lab Anim Res. 2007;23:127–131. [Google Scholar]
- 5.Slomiany BL, Piotrowski J, Slomiany A. Induction of tumor necrosis factor-alpha and apoptosis in gastric mucosal injury by indomethacin: effect of omeprazole and ebrotidine. Scand J Gastroenterol. 1997;32(7):638–642. doi: 10.3109/00365529708996511. [DOI] [PubMed] [Google Scholar]
- 6.Filaretova L, Tanaka A, Miyazawa T, Kato S, Takeuchi K. Mechanisms by which endogenous glucocorticoid protects against indomethacin-induced gastric injury in rats. Am J Physiol Gastrointest Liver Physiol. 2002;283(5):G1082–G1089. doi: 10.1152/ajpgi.00189.2002. [DOI] [PubMed] [Google Scholar]
- 7.Cao H, Wang MW, Jia JH, Wang QG, Cheng MS. Comparison of the effects of pantoprazole enantimers on gastric mucosal lesions and gastric epithelial cells in rats. J Health Sci. 2004;50:1–8. [Google Scholar]
- 8.Rao ChV, Ojha SK, Radhakrishnan K, Govindarajan R, Rastogi S, Mehrotra S, Pushpangadan P. Antiulcer activity of Utleria salicifolia rhizome extract. J Ethnopharmacol. 2004;91(2-3):243–249. doi: 10.1016/j.jep.2003.12.020. [DOI] [PubMed] [Google Scholar]
- 9.Kim YR, Lee MR, Kim YH, Jang BJ, Park SC, Han SH, Kim BH, Ryoo ZY, Kim KS. Effect of Opuntiahumifusa extract on indomethacin-induced gastric ulcer in Sprague Dawley rat. Lab Anim Res. 2005;21:375–578. [Google Scholar]
- 10.Raffin RP, Colomé LM, Schapoval EE, Jornada DS, Pohlmann AR, Guterres SS. Gastro-resistant microparticles containing sodium pantoprazole: stability and in vivo anti-ulcer activity. Open Drug Deliv J. 2007;1:28–35. [Google Scholar]
- 11.Dias PC, Foglio MA, Possenti A, de Carvalho JE. Antiulcerogenic activity of crude hydroalcoholic extract of Rosmarinus officinalis L. J Ethnopharmacol. 2000;69(1):57–62. doi: 10.1016/s0378-8741(99)00133-6. [DOI] [PubMed] [Google Scholar]
- 12.Cantarella G, Martinez G, Cutuli VM, Loreto C, D'Alcamo M, Prato A, Amico-Roxas M, Bernardini R, Clementi G. Adrenomedullin modulates COX-2 and HGF expression in reserpine-injuried gastric mucosa in the rat. Eur J Pharmacol. 2005;518(2-3):221–226. doi: 10.1016/j.ejphar.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Cantarella G, Martinez G, Di Benedetto G, Loreto C, Musumeci G, Prato A, Lempereur L, Matera M, Amico-Roxas M, Bernardini R, Clementi G. Protective effects of amylin on reserpine-induced gastric damage in the rat. Pharmacol Res. 2007;56(1):27–34. doi: 10.1016/j.phrs.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Işbil Büyükcoşkun N, Gulec G, Ozluk K. Protective effect of centrally-injected glucagon-like peptide-1 on reserpine-induced gastric mucosal lesions in rat: possible mechanisms. Turk J Gastroenterol. 2006;17(1):1–6. [PubMed] [Google Scholar]
- 15.Pope AJ, Toseland CD, Rushant B, Richardson S, McVey M, Hills J. Effect of potent urease inhibitor, fluorofamide, on Helicobacter sp. in vivo and in vitro. Dig Dis Sci. 1998;43(1):109–119. doi: 10.1023/a:1018884322973. [DOI] [PubMed] [Google Scholar]
- 16.Hahm KB, Kim DH, Lee KM, Lee JS, Surh YJ, Kim YB, Yoo BM, Kim JH, Joo HJ, Cho YK, Nam KT, Cho SW. Effect of long-term administration of rebamipide on Helicobacter pylori infection in mice. Aliment Pharmacol Ther. 2003;18:24–38. doi: 10.1046/j.1365-2036.18.s1.3.x. [DOI] [PubMed] [Google Scholar]
- 17.Aristoteli LP, O'Rourke JL, Danon S, Larsson H, Mellgard B, Mitchell H, Lee A. Urea, fluorofamide, and omeprazole treatments alter helicobacter colonization in the mouse gastric mucosa. Helicobacter. 2006;11(5):460–468. doi: 10.1111/j.1523-5378.2006.00439.x. [DOI] [PubMed] [Google Scholar]
- 18.Coghlan JG, Gilligan D, Humphries H, McKenna D, Dooley C, Sweeney E, Keane C, O'Morain C. Campylobacter pylori and recurrence of duodenal ulcers--a 12-month follow-up study. Lancet. 1987;2(8568):1109–1111. doi: 10.1016/s0140-6736(87)91545-5. [DOI] [PubMed] [Google Scholar]
- 19.Graham DY, Evans DG, Evans DJ., Jr Campylobacter pylori. The organism and its clinical relevance. J Clin Gastroenterol. 1989;11:43–48. [PubMed] [Google Scholar]
- 20.Wotherspoon AC, Ortiz-Hidalgo C, Falzon MR, Isaacson PG. Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. Lancet. 1991;338(8776):1175–1176. doi: 10.1016/0140-6736(91)92035-z. [DOI] [PubMed] [Google Scholar]
- 21.Cover TL, Blaser MJ. Helicobacter pylori and gastroduodenal disease. Annu Rev Med. 1992;43:135–145. doi: 10.1146/annurev.me.43.020192.001031. [DOI] [PubMed] [Google Scholar]
- 22.Lee A, Fox J, Hazell S. Pathogenicity of Helicobacter pylori: a perspective. Infect Immun. 1993;61(5):1601–1610. doi: 10.1128/iai.61.5.1601-1610.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marshall BJ. Helicobacter pylori in peptic ulcer: have Koch's postulates been fulfilled. Ann Med. 1995;27(5):565–568. doi: 10.3109/07853899509002470. [DOI] [PubMed] [Google Scholar]
- 24.Hahm KB, Kim DH, Lee KM, Lee JS, Surh YJ, Kim YB, Yoo BM, Kim JH, Joo HJ, Cho YK, Nam KT, Cho SW. Effect of long-term administration of rebamipide on Helicobacter pylori infection in mice. Aliment Pharmacol Ther. 2003;18:24–38. doi: 10.1046/j.1365-2036.18.s1.3.x. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Z, Liu ZQ, Zheng PY, Tang FA, Yang PC. Influence of efflux pump inhibitors on the multidrug resistance of Helicobacter pylori. World J Gastroenterol. 2010;16(10):1279–1284. doi: 10.3748/wjg.v16.i10.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takeuchi K, Konaka A, Nishijima M, Kato S, Yasuhiro T. Effects of pantoprazole, a novel H+/K+-ATPase inhibitor, on duodenal ulcerogenic and healing responses in rats: a comparative study with omeprazole and lansoprazole. J Gastroenterol Hepatol. 1999;14(3):251–257. doi: 10.1046/j.1440-1746.1999.01843.x. [DOI] [PubMed] [Google Scholar]
- 27.Cao H, Wang MW, Sun LX, Ikejima T, Hu ZQ, Zhao WH. Pharmacodynamic comparison of pantoprazole enantiomers: inhibition of acid-related lesions and acid secretion in rats and guinea-pigs. J Pharm Pharmacol. 2005;57(7):923–927. doi: 10.1211/0022357056361. [DOI] [PubMed] [Google Scholar]
- 28.Konturek SJ, Brzozowski T, Radecki T. Protective action of omeprazole, a benzimidazole derivative, on gastric mucosal damage by aspirin and ethanol in rats. Digestion. 1983;27(3):159–164. doi: 10.1159/000198946. [DOI] [PubMed] [Google Scholar]
- 29.Murakami I, Satoh H, Asano S, Maeda R. Role of capsaicin-sensitive sensory neurons and nitric oxide in the protective effect of lansoprazole, a proton pump inhibitor, on the gastric mucosa in rats. Jpn J Pharmacol. 1996;72(2):137–147. doi: 10.1254/jjp.72.137. [DOI] [PubMed] [Google Scholar]
- 30.Cao H, Wang MW, Jia JH, Wang QG, Cheng MS. Comparison of the effects of pantoprazole enantimers on gastric mucosal lesions and gastric epithelial cells in rats. J Health Sci. 2004;50:1–8. [Google Scholar]
- 31.Daw MA, Deegan P, Leen E, O'Moráin C. Short report: the effect of omeprazole on Helicobacter pylori and associated gastritis. Aliment Pharmacol Ther. 1991;5(4):435–439. doi: 10.1111/j.1365-2036.1991.tb00047.x. [DOI] [PubMed] [Google Scholar]
- 32.Iwahi T, Satoh H, Nakao M, Iwasaki T, Yamazaki T, Kubo K, Tamura T, Imada A. Lansoprazole, a novel benzimidazole proton pump inhibitor, and its related compounds have selective activity against Helicobacter pylori. Antimicrob Agents Chemother. 1991;35(3):490–496. doi: 10.1128/aac.35.3.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hunt RH. Eradication of H. pylori infection. Am J Med. 1996;100:42–51. doi: 10.1016/s0002-9343(96)80228-2. [DOI] [PubMed] [Google Scholar]
- 34.Mobley HL, Hausinger RP. Microbial ureases: significance, regulation, and molecular characterization. Microbiol Rev. 1989;53(1):85–108. doi: 10.1128/mr.53.1.85-108.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shin JH, Yang M, Nam SW, Kim JT, Myung NH, Bang WG, Roe IH. Use of egg yolk-derived immunoglobulin as an alternative to antibiotic treatment for control of Helicobacter pylori infection. Clin Diagn Lab Immunol. 2002;9(5):1061–1066. doi: 10.1128/CDLI.9.5.1061-1066.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shin JH, Roe IH, Kim HG. Production of anti-Helicobacter pylori urease-specific immunoglobulin in egg yolk using an antigenic epitope of H. pylori urease. J Med Microbiol. 2004;53:31–34. doi: 10.1099/jmm.0.05327-0. [DOI] [PubMed] [Google Scholar]
- 37.Malekshahi ZV, Gargari SL, Rasooli I, Ebrahimizadeh W. Treatment of Helicobacter pylori infection in mice with oral administration of egg yolk-driven anti-UreC immunoglobulin. Microb Pathog. 2011;51(5):366–372. doi: 10.1016/j.micpath.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 38.Attallah AM, Abbas AT, Ismail H, Abdel-Raouf M, El-Dosoky I. Efficacy of passive immunization with IgY antibodies to a 58-kDa H. pylori antigen on severe gastritis in BALB/c mouse model. J Immunoassay Immunochem. 2009;30(4):359–377. doi: 10.1080/15321810903187922. [DOI] [PubMed] [Google Scholar]
- 39.Nomura S, Suzuki H, Masaoka T, Kurabayashi K, Ishii H, Kitajima M, Nomoto K, Hibi T. Effect of dietary anti-urease immunoglobulin Y on Helicobacter pylori infection in Mongolian gerbils. Helicobacter. 2005;10(1):43–52. doi: 10.1111/j.1523-5378.2005.00290.x. [DOI] [PubMed] [Google Scholar]
- 40.Lee KA, Chang SK, Lee YJ, Lee JH, Koo NS. Acid stability of anti-Helicobacter pyroli IgY in aqueous polyol solution. J Biochem Mol Biol. 2002;35(5):488–493. doi: 10.5483/bmbrep.2002.35.5.488. [DOI] [PubMed] [Google Scholar]
- 41.Shin JH, Nam SW, Kim JT, Yoon JB, Bang WG, Roe IH. Identification of immunodominant Helicobacter pylori proteins with reactivity to H. pylori-specific egg-yolk immunoglobulin. J Med Microbiol. 2003;52:217–222. doi: 10.1099/jmm.0.04978-0. [DOI] [PubMed] [Google Scholar]
- 42.Kazimierczuk K, Cova L, Ndeboko B, Szczyrk U, Targosz A, Brzozowski T, Sirko A. Genetic immunization of ducks for production of antibodies specific to Helicobacter pylori UreB in egg yolks. Acta Biochim Pol. 2005;52(1):261–266. [PubMed] [Google Scholar]
- 43.Horie K, Horie N, Abdou AM, Yang JO, Yun SS, Chun HN, Park CK, Kim M, Hatta H. Suppressive effect of functional drinking yogurt containing specific egg yolk immunoglobulin on Helicobacter pylori in humans. J Dairy Sci. 2004;87(12):4073–4079. doi: 10.3168/jds.S0022-0302(04)73549-3. [DOI] [PubMed] [Google Scholar]
- 44.Suzuki H, Nomura S, Masaoka T, Goshima H, Kamata N, Kodama Y, Ishii H, Kitajima M, Nomoto K, Hibi T. Effect of dietary anti-Helicobacter pylori-urease immunoglobulin Y on Helicobacter pylori infection. Aliment Pharmacol Ther. 2004;20:185–192. doi: 10.1111/j.1365-2036.2004.02027.x. [DOI] [PubMed] [Google Scholar]
- 45.Pope AJ, Toseland CD, Rushant B, Richardson S, McVey M, Hills J. Effect of potent urease inhibitor, fluorofamide, on Helicobacter sp. in vivo and in vitro. Dig Dis Sci. 1998;43(1):109–119. doi: 10.1023/a:1018884322973. [DOI] [PubMed] [Google Scholar]