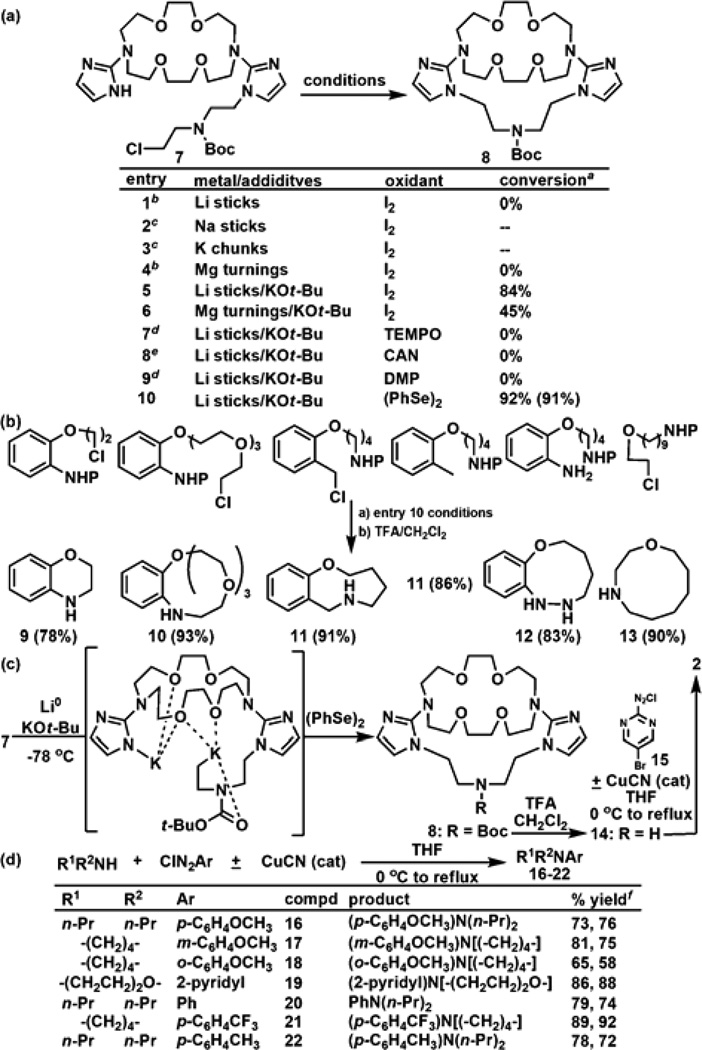
Figure 3.
(a) The conditions shown in entry 10 used Li sticks/KOt-Bu and (PhSe)2 to afford 8 in an isolated yield of 91% yield. (b) Utility of this oxidative cyclization is demonstrated in the syntheses of cyclic secondary amines 9–13 (P = Boc). (c) Synthesis of ionophore 2 featuring an oxidative C-N bond forming cyclization and either a Sandmeyer-like reaction (+CuOAc) or a metal-free aminoarylation (−CuOAc) with a secondary amine. (d) Utility of these aminoarylation conditions used in Figure 2c is demonstrated in the synthesis of tertiary arylamines 16–22. Footnotes: aDetermined by LCMS. Isolated yield shown in parenthesis. bMajor product was a dimer. cStarting material recovered. dMajor product was an alkene. eMajor product was an alkane. fFirst/second values rerpresent the % yield obtained with/without CuOAc (cat).