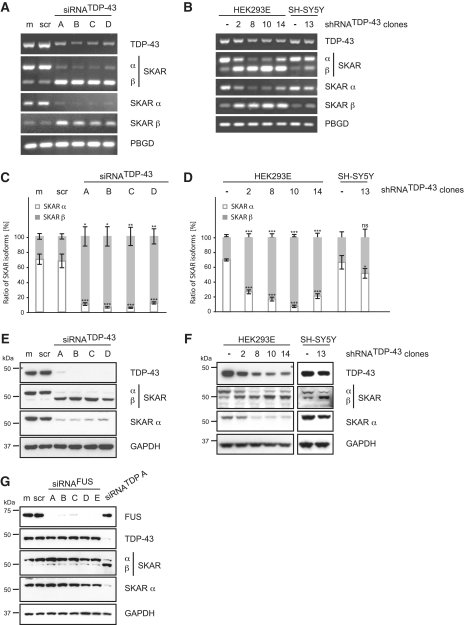
Figure 2.
Validation of SKAR alternative splicing upon transient silencing of TDP-43. TDP-43 was either silenced transiently by siRNA treatment (A, C, E and G) or stably by use of lentiviral particles encoding for a TDP-43-specific shRNA followed by the selection of single cell clones (B, D and F). For transient silencing, HEK293E cells were either mock treated (m) or transiently transfected with scrambled control siRNA (scr), with one of four different TDP-43-specific siRNAs (siRNATDP-43 A-D) or with one of five specific siRNAs against FUS (siRNAFUS A-E), as indicated. (A–D) Total RNA was extracted and analyzed by RT–PCR. (A and B) Semi-quantitative RT–PCR was performed with primer pairs specific for TDP-43, SKAR (ex2–ex4), SKAR α (ex2|3–ex4) and SKAR β (ex2|4–ex4). (C and D) Real-time PCR was performed with primer pairs against SKAR α (ex2|3–ex4) (white bars), SKAR β (ex2|4–ex4) (gray bars) and total SKAR (ex5|6–ex7). PBGD was used as a housekeeping gene. Resulting relative SKARα/PBGD, SKARβ/PBGD and total SKAR/PBGD ratios were recalculated into absolute copy values and normalized to total SKAR values. Shown are the mean values of five independent experiments ± SEM. *P < 0.05; **P < 0.005; ***P < 0.0005; ns = not significant. Original qRT–PCR data is presented in Supplementary Figure S1A and S1B, respectively. (E–G) Protein was extracted, electrophoresed and resulting western blots probed with antibodies specific for TDP-43, SKAR (both isoforms) and SKAR α. GAPDH was used as a loading control. FUS silencing efficiency was controlled by use of an anti-FUS antibody. Note, that, depending on the primer pair and antibody used, SKAR RNA and protein isoforms, respectively, are visualized as two bands with different molecular weights. The upper band represents SKAR α, the lower corresponds to SKAR β, as indicated.