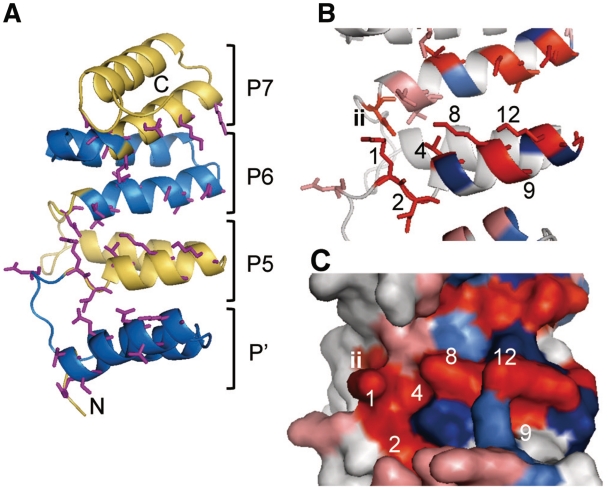
Figure 4.
Structural model of HCF152. (A) Cartoon diagram of the model of the 148 aa from HCF152 containing a PPR-like helical structure (P′) and three PPR motifs (5th, 6th and 7th PPR motifs; P5-P7). The helical repeats are colored alternately in blue or yellow. The side chains of aa involved in the RNA interaction are shown by sticks (1st, 2nd, 4th, 8th, 9th, 12th and ‘ii’ aa). N and C indicate the N- and C-terminus, respectively. (B) Magnification of the model containing the 5th and 6th PPR motif. The numbers of residues in the 5th motif are shown. Residue for which mutations had reduced RNA binding activity are colored in red (1st, 2nd, 4th, 8th, 9th, 12th, 14th and ‘ii’ aa; or salmon pink for the corresponding position, but not experimentally determined). Residues for which mutations had no effect (5th, 11th and 13th aa) are displayed in blue (or light blue for the corresponding position), respectively. (C) Surface representation of the model. The numbers and colors of the residues are the same as in (B).