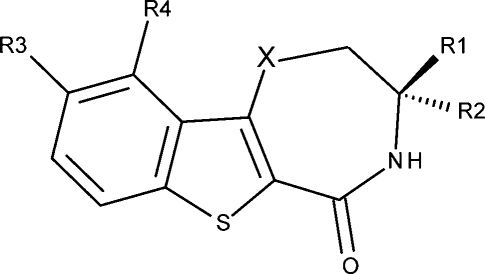
Table 1. Benzothiophene Analogues: Structures, Considered Tautomers and Species, and MK2 Inhibition IC50 Values (M).28,29.
| considered |
log(1/IC50) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| ligand no. | X | R1 | R2 | R3 | R4 | tautomers | species | exp | calcd |
| 1 | NH | H | H | OCH3 | H | T1,T2 | S1,S5,S7 | 6.745 | 6.739 |
| 2 | NH | CH3 | H | OCH3 | H | T1,T2 | S1,S5,S7 | 7.398 | 7.635 |
| 3 | NH | H | CH3 | OCH3 | H | T1,T2 | S1,S5,S7 | 6.523 | 6.713 |
| 4 | NH | (CH2)2CH3 | H | OCH3 | H | T1,T2 | S1,S5,S7 | 5.818 | 5.486 |
| 5 | NH | CH2OH | H | OCH3 | H | T1,T2,T5 | S1,S5,S7,S11 | 7.854 | 7.446 |
| 6 | S | H | H | OCH3 | H | T1,T3 | S1,S9 | 5.963 | 5.547 |
| 7 | CH2 | H | H | OCH3 | H | T1,T3 | S1,S9 | 6.301 | 6.281 |
| 8 | NH | CH2NH2 | H | OCH3 | H | T1,T2 | S1,S2,S5,S6 | 8.301 | 8.436 |
| 9 | NH | CH2NHCH2CH3 | H | OCH3 | H | T1,T2 | S1,S2,S5,S6 | 6.244 | 6.021 |
| 10 | NH | CH2NH(CH2)2CH3 | H | OCH3 | H | T1,T2 | S1,S2,S5,S6 | 6.347 | 6.621 |
| 11 | NH | CH2NHCH2Pha | H | OCH3 | H | T1,T2,T5 | S1,S2,S5,S6,S12 | 6.244 | 6.418 |
| 12 | NH | CH2N(CH3)CH2Ph | H | OCH3 | H | T1,T2,T5 | S1,S2,S5–S7,S12 | 5.719 | 6.074 |
| 13 | NH | CH3 | H | OH | H | T1,T2 | S1,S2,S5,S6–S8 | 6.721 | 6.944 |
| 14 | NH | CH3 | H | OCH2(3-OCH3-Ph) | H | T1,T2 | S1,S5,S7 | 6.959 | 7.022 |
| 15 | NH | CH3 | H | OCH2-c-C3H5 | H | T1,T2 | S1,S5,S7 | 5.879 | 5.874 |
| 16 | NH | CH3 | H | O(CH2)2OCH3 | H | T1,T2 | S1,S5,S7 | 6.569 | 6.769 |
| 17 | NH | CH3 | H | OCH2CH(CH3)2 | H | T1,T2 | S1,S5,S7 | 6.398 | 6.238 |
| 18 | NH | CH3 | H | OCH2COOCH3 | H | T1,T2 | S1,S5,S7 | 7.523 | 7.212 |
| 19 | NH | CH3 | H | OCH2-c-C6H11 | H | T1,T2 | S1,S5,S7 | 5.463 | 5.593 |
| 20 | NH | CH3 | H | O–CH=CHb | T1,T2 | S1,S5,S7 | 7.796 | 7.775 | |
| 21 | NH | CH3 | H | O–CH2–CH2 | T1,T2 | S1,S5,S7 | 7.553 | 7.593 | |
| 22 | NH | CH3 | H | N=CH–CH=CH | T1,T2,T4 | S1,S5,S7,S10 | 9.000 | 8.549 | |
| 23 | NH | CH3 | H | OC(Ph)=CH | T1,T2 | S1,S5,S7 | 6.824 | 6.801 | |
| 24 | NH | CH3 | H | N=C(Cl)-CH=CH | T1,T2,T4 | S1,S5,S7,S10 | 8.699 | 8.474 | |
| 25 | NH | CH3 | H | N=C(Ph)-CH=CH | T1,T2,T4 | S1,S5,S7,S10 | 8.046 | 7.758 | |
| 26 | NH | CH3 | H | N=C(4-Py)-CH=CHc | T1,T2,T4 | S1,S5–S7,S10 | 7.854 | 7.967 | |
| 27 | NH | CH3 | H | N=C(3-Py)-CH=CH | T1,T2,T4 | S1,S5–S7,S10 | 8.301 | 7.705 | |
| 28 | NH | CH3 | H | N=C(5-Pm)-CH=CHd | T1,T2,T4 | S1,S5,S7,S10 | 7.638 | 7.356 | |
| 29 | NH | CH3 | H | N=C(2-OCH3-Ph)-CH=CH | T1,T2 | S1,S5,S7 | 7.699 | 7.517 | |
| 30 | NH | CH3 | H | N=C(3-OCH3-Ph)-CH=CH | T1,T2,T4 | S1,S5,S7,S10 | 7.387 | 7.199 | |
| 31 | NH | CH3 | H | N=C(2-F-Ph)-CH=CH | T1,T2,T4 | S1,S5,S7,S10 | 7.854 | 7.705 | |
| 32 | NH | CH3 | H | N=C(2-CH3-Ph)-CH=CH | T1,T2,T4 | S1,S5,S7,S10 | 7.523 | 7.771 | |
| 33 | NH | CH3 | H | N=C(4-CH3-3-Py)-CH=CH | T1,T2,T4 | S1,S2,S5–S7,S10 | 8.301 | 8.265 | |
| 34 | NH | CH2N-Tpe | H | OCH3 | H | T1,T2 | S1,S2,S5,S6 | <4.699 | 4.879 |
| 35 | NH | CH2N-Mof | H | OCH3 | H | T1,T2,T5 | S1,S2,S5,S7,S11 | <4.699 | 4.812 |
Ph: phenyl.
R3 and R4 join the phenyl ring to form cyclic derivatives in ligands 20–33.
Py: pyridyl.
Pm: pyrimidinyl.
Tp: tetrahydropyrrolyl.
Mo: morpholinyl.