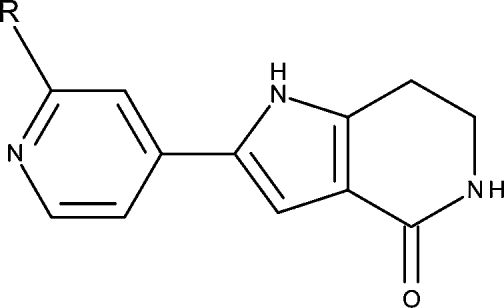
Table 2. Pyrrolopyridine Analogues: Structures, Considered Species,a and MK2 Enzyme Inhibition IC50 Values (M)27.
| log(1/IC50) |
||||
|---|---|---|---|---|
| ligand no. | R | considered species | exp | calcd |
| 36 | H | S1,S3,S5 | 6.767 | 6.561 |
| 37 | Phb | S1,S3,S5 | 7.180 | 7.520 |
| 38 | 4-Pyc | S1,S2,S3,S5 | 7.252 | 7.339 |
| 39 | 3-Py | S1,S3,S5 | 7.319 | 7.486 |
| 40 | 3-Thd | S1,S3,S5 | 7.119 | 7.550 |
| 41 | 2-Npe | S1,S3,S5 | 7.284 | 6.934 |
| 42 | 2-Btf | S1,S3,S5 | 7.523 | 7.347 |
| 43 | 3-Qg | S1,S3,S5 | 8.071 | 7.994 |
| 44 | 2-OH-Ph | S1,S2,S3–S5 | 6.387 | 6.920 |
| 45 | 3-OH-Ph | S1,S2,S3–S5 | 7.602 | 7.829 |
| 46 | 4-OH-Ph | S1,S2,S3–S5 | 7.678 | 7.669 |
| 47 | 2-Cl-Ph | S1,S3,S5 | 6.218 | 6.430 |
| 48 | 3-Cl-Ph | S1,S3,S5 | 7.432 | 7.747 |
| 49 | 4-Cl-Ph | S1,S3,S5 | 7.495 | 7.568 |
| 50 | 2-F-Ph | S1,S3,S5 | 6.900 | 7.165 |
| 51 | 3-F-Ph | S1,S3,S5 | 7.523 | 7.659 |
| 52 | 4-F-Ph | S1,S3,S5 | 7.301 | 7.422 |
| 53 | 4-CF3-Ph | S1,S3,S5 | 7.149 | 7.181 |
| 54 | 4-COCH3-Ph | S1,S3,S5 | 7.292 | 7.228 |
| 55 | 4-OCH3-Ph | S1,S3,S5 | 7.260 | 7.205 |
| 56 | 4-NH2-Ph | S1,S3,S5 | 7.387 | 7.340 |
| 57 | 4-[CONH-c-C5H9]-Ph | S1,S3,S5 | 8.097 | 8.153 |
| 58 | 4-[CONH-c-C6H11]-Ph | S1,S3,S5 | 7.770 | 7.662 |
| 59 | 4-[CONHCH2Ph]-Ph | S1,S3,S5 | 8.097 | 7.786 |
| 60 | 4-[CONH(CH2)2Ph]-Ph | S1,S3,S5 | 7.337 | 7.508 |
| 61 | 4-[CONH(CH3)CH2Ph]-Ph | S1,S3,S5 | 7.252 | 7.309 |
| 62 | Cl | S1,S3,S5 | 6.216 | 6.270 |
| 63 | 5-Pmh | S1,S3,S5 | 7.081 | 7.115 |
| 64 | 4-CN-Ph | S1,S3,S5 | 7.208 | 7.218 |
| 65 | 4-COOH-Ph | S2,S3,S5 | 7.658 | 7.683 |
| 66 | 4-[CONH-c-C3H5]-Ph | S1,S3,S5 | 7.824 | 7.816 |
All compounds present as tautomers T1 and T2.
Phenyl.
Pyridinyl.
Thienyl.
Naphthyl (marked as 3-Np in the original publication).
Benzothienyl.
Quinolinyl.
Pyrimidinyl.