Figure 2. Heat map analysis of identified peptides.
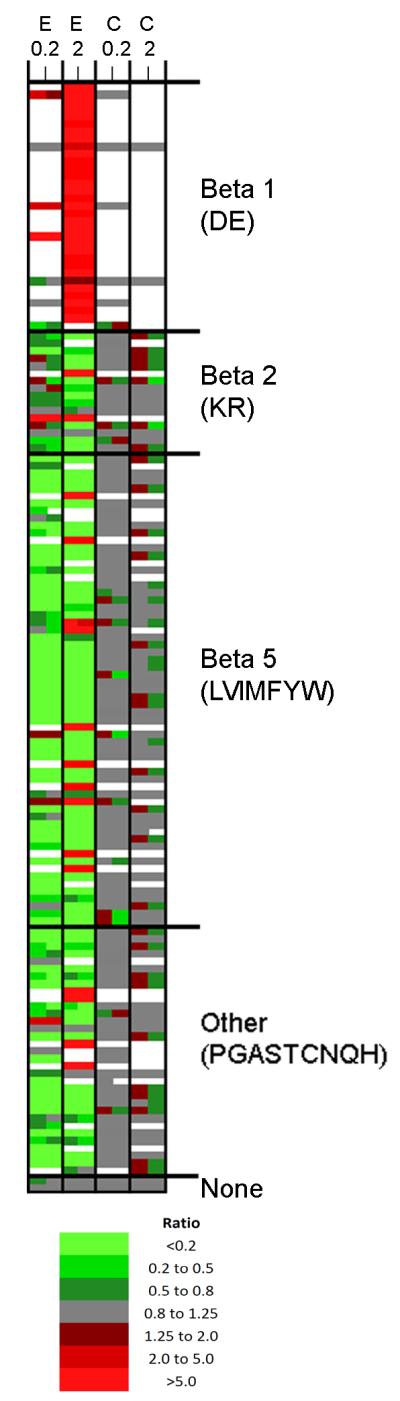
The 147 different peptides identified by MS/MS sequence analysis were plotted as separate rows; for this analysis, peptides found in two or more different charge states were averaged together. The individual replicates for each experiment are indicated in separate columns. E, epoxomicin; C, control. The controls for the 0.2 and 2 μM epoxomicin experiments are indicated separately; all four of these replicates represent similar controls. The ratio was color coded using the scheme shown in the bottom of the figure, with green representing decreases and red representing increases, relative to the average of the untreated control samples. White represents peptides that were not detected, or which were detected but showed peak overlap with another peptide that prevented accurate quantification of the relative level. The various peptides were sorted by cleavage site required to generate the observed peptide; beta-1 sites (with D or E in the P1 position), beta-2 (K or R in this position), beta-5 (L, V, I, M, F, Y, or W in this position), or other amino acids that are neither acidic, basic, or hydrophobic (P, G, A, S, T, C, N, Q, or H), and which may be cleaved by the beta-5 subunit. Two peptides corresponded to small proteins (thymosin beta-4 and beta-10) that did not require any cleavage, other than removal of the N-terminal initiation methionine (which was not considered in this analysis as a cleavage). For this analysis, only half of the peptides represented N- or C-terminal protein fragments, which required a single cleavage to generate the peptide. For the internal peptides that required two cleavages, if either side was an acidic residue, it was grouped in the beta-1 (DE) group. For the remaining peptides, if either side was a basic residue it was placed in the beta-2 (KR) group. Of the remaining peptides, only those with a hydrophobic group on both sides were placed in the beta-5 (LVIMFYW) group, and the remainder were placed in the “other” group. Names of proteins, peptide sequences, and other information including the values of the ratios and statistical calculations are included in supplemental Table S2.
