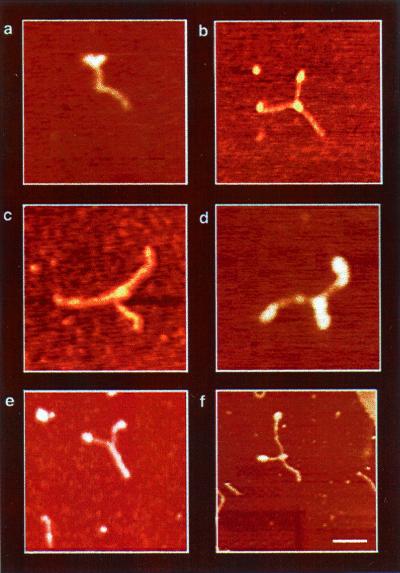
Figure 4.
AFM images of DbpA–RNA complexes in the presence of ATP (DbpA–RNA–ATP). The Y structure, observed in all images, represents the most dominant intermediate trapped during unwinding catalysis. It comprises two single ssRNA regions at the arms and dsRNA at the trunk of the Y. Protein signals were observed as globular structures at the fork junction (a--d and f) and on the tips of the Y-shaped arms (a, b, d, and f). Because of the structure of the RNA construct, two proteins can sometimes be seen entering the dsRNA region from opposite sides, as shown in d. On the other hand, e shows a Y-shaped molecule without a protein signal at the fork junction. The formation of this complex may result from substrate reannealing, or alternatively, the central protein may be washed away during sample preparations. The measured end-to-end contour length of the Y-shaped molecules shows a significant elongation (see Fig. 5c) because of the ssRNA formation during unwinding catalysis. In addition, the variation in the amount of unwinding among the Y-shaped complexes are snapshots representing the distribution of structural conformations during catalysis.