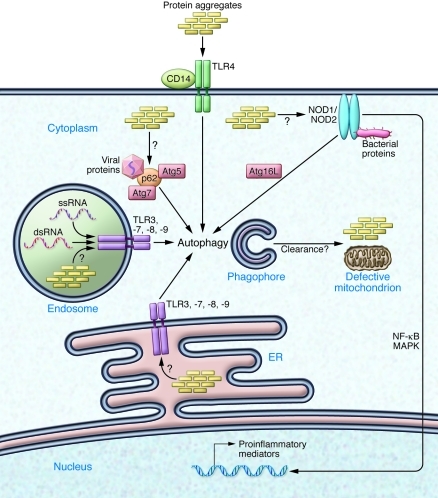
Figure 2. Innate immune receptors as sensors of intraneuronal distress.
Neurons express innate immune receptors that serve as sensors of danger signals. TLRs may recognize endogenous molecules and protein aggregates such as Aβ assemblies, ssRNA, or dsRNA aside from molecules associated with pathogens. While TLR4 and its co-receptor CD14 are present at the cell surface, TLR3, -7, -8, and -9 are located in the ER and endosomal compartments. Activation of TLRs can lead to initiation of autophagy via TRIF/RIP1 and possibly induce the clearance of defective organelles or protein aggregates. Activation of NOD1 or NOD2 may result in NF-κB–mediated transcription of pro-inflammatory genes or initiation of autophagy via Atg16L. The adaptor protein p62 can detect viral proteins in neurons and initiate clearance of viral particles via autophagy involving Atg5 and Atg7. It may also assist in the clearance of abnormal protein aggregates. Atg, autophagy-related protein; NOD, nucleotide binding oligomerization domain-like; p62, nucleoporin 62; TRIF, TIR domain–containing adapter-inducing IFN-β; RIP1, receptor interacting protein-1.