Cyclin E, together with cyclin-dependent kinase 2 (CDK2), functions as a gatekeeper to promote G1/S transitions and the initiation of DNA replication. In normal cells, cyclin E–associated kinase activity is exquisitely regulated, with activity being limited to a brief time interval between late G1 and early S phase. Human cancers frequently exhibit deregulated cyclin E–associated kinase activity resulting from overexpression of cyclin E and loss of cyclin-dependent kinase inhibition (via p53 mutations) promoting genetic instability and cell proliferation [1]. Increased levels of cyclin E correlate with tumorigenesis and are a poor prognostic indicator independent of proliferation rate, suggesting that cyclin E's role in tumorigenesis is not limited to promoting increased cell proliferation [2], [3]. By eliminating regulatory constraints using p53 null cells, we and others have shown that overexpression or endogenous expression of stabilizing mutant forms of cyclin E can lead to hyperproliferation, genetic instability, and malignancy in cell culture and murine models [4], [5]. Normal cells suppress the effects of excess/stabilized cyclin E via the G1/S checkpoint involving the p53/p21 pathway.
Five isoforms of cyclin E, ranging in size from 33 to 44 kDa, have been identified in tumors over-expressing cyclin E. These low molecular weight forms of cyclin E (LMW-E) are generated through post-translational cleavage of full-length cyclin E by the elastase family of serine proteases in tumor cells [6], [7]. In comparison to full-length cyclin E (50 kDa), LMW-E forms are uniquely expressed in tumor cells, exhibit enhanced CDK2-associated kinase activity, have increased affinity for CDK2 [7]–[9], and exhibit decreased inhibition by CDK2 inhibitors, p21 and p27 (Figure 1) [10], [11]. Ectopic expression of LMW-E isoforms promotes cell proliferation, genetic instability, centrosome amplification, and malignancy [12], [13]. In addition, clinical studies have shown that high LMW-E is strongly associated with poor survival in breast cancer [2], colorectal cancer [14], [15], ovarian cancer [16], and melanomas [17]. Given its unique properties and distinct function in human cancers, targeting LMW-E could have important therapeutic implications.
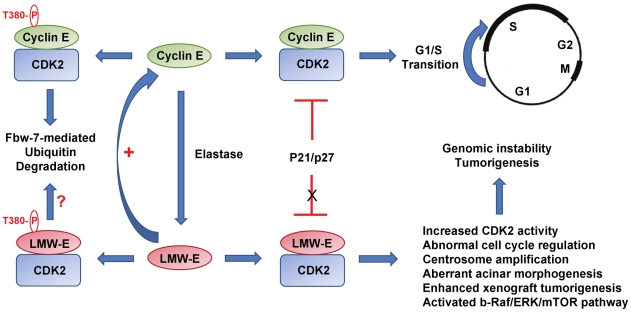
Figure 1. Low molecular weight cyclin E promotes tumorigenesis.
In normal cells, cyclin E/CDK2 is tightly regulated and triggers the onset of S phase. In tumors, cyclin E undergoes proteolytic processing generating low molecular weight species that exhibit increased kinase activity and resistance to inhibition by cyclin kinase inhibitors p21/p27. The expression of LMW-E promotes aberrant acinar morphogenesis, centrosome amplification, and tumors associated with activation of the bRaf/ERK/mTOR pathway. LMW-E, low molecular weight cyclin E.
The study by Duong et al. in this issue of PLoS Genetics [18] convincingly uncovers the tumorigenic potential of LMW-E. The authors used three different model systems—3D acinar cultures, xenograft transplantation, and transgenic mice—to show that overexpression of LMW-E is sufficient to induce aberrant acinar morphogenesis in culture and mammary tumors in mice (Figure 1). When grown on Matrigel, immortalized human mammary epithelial cells (hMECs) expressing LMW-E exhibit large misshapen multiacinar structures resulting from defective growth arrest and apoptosis that mimic morphologic features of breast carcinomas. Further, ectopic expression of LMW-E in immortalized hMECs promotes tumorigenesis in xenografts and transgenic mice to a much greater extent than full-length cyclin E. Consistent with the reports by Akli et al. and Nanos-Webb et al., tumorigenesis associated with LMW-E is dependent on CDK2 [19], [20]. Furthermore, in vivo passaging of tumor cells increases the expression of LMW-E, suggesting that LMW-E provides a selective growth advantage to the tumor. Duong et al. also took advantage of a proteomic analysis termed reverse-phase protein array assay (RPPA) to examine protein expression patterns in cultured tumor cells and in breast tumors expressing high LMW-E levels. Their analyses revealed that multiple components of the b-RAF-ERK1/2-mTOR pathway are elevated in these cells. Activation of the b-RAF-ERK1/2-mTOR pathway normally promotes cell division and cell survival. Consistent with this, the authors observed that endogenous cyclin E levels are also increased in cells expressing high LMW-E, indicative of the existence of a positive feedback loop that promotes cell proliferation. Both high LMW-E levels and up-regulation of the b-RAF-ERK1/2-mTOR signaling pathway are associated with poor survival, suggesting functional correlation of these events in aggressive tumors. Importantly, the authors demonstrated that combination therapy targeting LMW-E/CDK2 and the b-RAF-ERK1/2-mTOR pathway has a synergistic effect in abrogating the tumorigenic effect of LMW-E. Thus, the identification of these downstream regulators may provide novel biomarkers and/or potential therapeutic targets for LMW-E–expressing tumors.
The report that LMW-E potentiates tumorigenesis in three independent model systems associated with activation of the b-RAF-ERK1/2-mTOR pathway is intriguing. However, there are many important questions about the role of LMW-E in tumorigenesis that need to be addressed. 1) What is the functional relationship between LMW-E and full-length cyclin E? In each tumor model reported by Duong et al., the effect was examined by over-expressing LMW-E in a background of endogenous full-length cyclin E. Further, the authors show that ectopic expression of LMW-E in transplanted xenografts triggers tumor evolution and results in increased levels of endogenous cyclin E. Thus, the contribution of endogenous full-length cyclin E in tumorigenesis cannot be excluded. In addition, Spruck et al. reported that the level of LMW-E correlates with full-length cyclin E, suggesting that LMW-E reflects the total cyclin E protein in primary breast tumors, cell lines, and even normal breast tissue [21]. To examine the effect of LMW-E in the absence of over-expression, and in the absence of full-length cyclin E, it will be important to use a knock-in model in which expression of LMW-E is driven from the endogenous cyclin E promoter. 2) What is the relationship between LMW-E and the b-Raf-ERK1/2-mTOR signaling pathway? The authors demonstrated that the b-Raf-ERK1/2-mTOR signaling pathway is activated in tumors expressing high levels of LMW-E. The b-Raf-ERK1/2-mTOR pathway may be a downstream signaling pathway deregulated by LMW-E, or it could be a parallel survival pathway selected in LMW-E–expressing tumors. In particular, the fact that only combinational therapy targeting both cyclin E–associated kinase activity and the b-Raf-ERK1/2-mTOR pathway generates an anti-tumor effect argues against a direct cause–effect relationship and is suggestive of a parallel pathway. 3) Is LMW-E expression required for tumor growth and does down-regulation of LMW-E alter tumor growth, invasion, or metastasis? The authors have generated an inducible model that should facilitate these studies. 4) Is the tumor-promoting activity of LMW-E due to enhanced deregulated kinase activity or to alternative target specificity? The LMW-E construct used in these studies has an N-terminal deletion (40 amino acids) that eliminates the proposed nuclear localization signal (NLS) and potentially affects the intracellular localization and substrate specificity [22], [23]. 5) How is LMW-E generated in tumors, and is it tumor-type specific? It has been proposed and demonstrated by Caruso et al. that many tumors have elevated protease activity and decreased levels of protease inhibitors such as elafin [24] that may contribute to the generation of LMW-E. Further characterization of the proteolytic pathways that target cyclin E in tumors may provide alternative therapeutic targets.
Footnotes
The authors have declared that no competing interests exist.
The authors received no specific funding for this article.
References
- 1.Hwang HC, Clurman BE. Cyclin E in normal and neoplastic cell cycles. Oncogene. 2005;24:2776–2786. doi: 10.1038/sj.onc.1208613. [DOI] [PubMed] [Google Scholar]
- 2.Keyomarsi K, Tucker SL, Buchholz TA, Callister M, Ding Y, et al. Cyclin E and survival in patients with breast cancer. N Engl J Med. 2002;347:1566–1575. doi: 10.1056/NEJMoa021153. [DOI] [PubMed] [Google Scholar]
- 3.Porter PL, Malone KE, Heagerty PJ, Alexander GM, Gatti LA, et al. Expression of cell-cycle regulators p27Kip1 and cyclin E, alone and in combination, correlate with survival in young breast cancer patients. Nat Med. 1997;3:222–225. doi: 10.1038/nm0297-222. [DOI] [PubMed] [Google Scholar]
- 4.Loeb KR, Kostner H, Firpo E, Norwood T, Tsuchiya KD, et al. A mouse model for cyclin E-dependent genetic instability and tumorigenesis. Cancer Cell. 2005;8:35–47. doi: 10.1016/j.ccr.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 5.Minella AC, Swanger J, Bryant E, Welcker M, Hwang H, et al. p53 and p21 form an inducible barrier that protects cells against cyclin E-cdk2 deregulation. Curr Biol. 2002;12:1817–1827. doi: 10.1016/s0960-9822(02)01225-3. [DOI] [PubMed] [Google Scholar]
- 6.Harwell RM, Porter DC, Danes C, Keyomarsi K. Processing of cyclin E differs between normal and tumor breast cells. Cancer Res. 2000;60:481–489. [PubMed] [Google Scholar]
- 7.Porter DC, Zhang N, Danes C, McGahren MJ, Harwell RM, et al. Tumor-specific proteolytic processing of cyclin E generates hyperactive lower-molecular-weight forms. Mol Cell Biol. 2001;21:6254–6269. doi: 10.1128/MCB.21.18.6254-6269.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harwell RM, Mull BB, Porter DC, Keyomarsi K. Activation of cyclin-dependent kinase 2 by full length and low molecular weight forms of cyclin E in breast cancer cells. J Biol Chem. 2004;279:12695–12705. doi: 10.1074/jbc.M313407200. [DOI] [PubMed] [Google Scholar]
- 9.Wingate H, Puskas A, Duong M, Bui T, Richardson D, et al. Low molecular weight cyclin E is specific in breast cancer and is associated with mechanisms of tumor progression. Cell Cycle. 2009;8:1062–1068. doi: 10.4161/cc.8.7.8119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akli S, Zheng PJ, Multani AS, Wingate HF, Pathak S, et al. Tumor-specific low molecular weight forms of cyclin E induce genomic instability and resistance to p21, p27, and antiestrogens in breast cancer. Cancer Res. 2004;64:3198–3208. doi: 10.1158/0008-5472.can-03-3672. [DOI] [PubMed] [Google Scholar]
- 11.Wingate H, Zhang N, McGarhen MJ, Bedrosian I, Harper JW, et al. The tumor-specific hyperactive forms of cyclin E are resistant to inhibition by p21 and p27. J Biol Chem. 2005;280:15148–15157. doi: 10.1074/jbc.M409789200. [DOI] [PubMed] [Google Scholar]
- 12.Akli S, Van Pelt CS, Bui T, Multani AS, Chang S, et al. Overexpression of the low molecular weight cyclin E in transgenic mice induces metastatic mammary carcinomas through the disruption of the ARF-p53 pathway. Cancer Res. 2007;67:7212–7222. doi: 10.1158/0008-5472.CAN-07-0599. [DOI] [PubMed] [Google Scholar]
- 13.Bagheri-Yarmand R, Biernacka A, Hunt KK, Keyomarsi K. Low molecular weight cyclin E overexpression shortens mitosis, leading to chromosome missegregation and centrosome amplification. Cancer Res. 2010;70:5074–5084. doi: 10.1158/0008-5472.CAN-09-4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou YJ, Xie YT, Gu J, Yan L, Guan GX, et al. Overexpression of cyclin E isoforms correlates with poor prognosis in rectal cancer. Eur J Surg Oncol. 2011;37:1078–1084. doi: 10.1016/j.ejso.2011.08.139. [DOI] [PubMed] [Google Scholar]
- 15.Corin I, Di Giacomo MC, Lastella P, Bagnulo R, Guanti G, et al. Tumor-specific hyperactive low-molecular-weight cyclin E isoforms detection and characterization in non-metastatic colorectal tumors. Cancer Biol Ther. 2006;5:198–203. doi: 10.4161/cbt.5.2.2356. [DOI] [PubMed] [Google Scholar]
- 16.Davidson B, Skrede M, Silins I, Shih Ie M, Trope CG, et al. Low-molecular weight forms of cyclin E differentiate ovarian carcinoma from cells of mesothelial origin and are associated with poor survival in ovarian carcinoma. Cancer. 2007;110:1264–1271. doi: 10.1002/cncr.22918. [DOI] [PubMed] [Google Scholar]
- 17.Bales E, Mills L, Milam N, McGahren-Murray M, Bandyopadhyay D, et al. The low molecular weight cyclin E isoforms augment angiogenesis and metastasis of human melanoma cells in vivo. Cancer Res. 2005;65:692–697. [PubMed] [Google Scholar]
- 18.Duong MT, Akli S, Wei C, Wingate HF, Liu W, et al. LMW-E/CDK2 deregulates acinar morphogenesis, induces tumorigenesis and associates with the activated b-Raf-ERK1/2-mTOR pathway in breast cancer patients. PLoS Genet. 2012;8:e1002538. doi: 10.1371/journal.pgen.1002538. doi: 10.1371/journal.pgen.1002538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akli S, Van Pelt CS, Bui T, Meijer L, Keyomarsi K. Cdk2 is required for breast cancer mediated by the low-molecular-weight isoform of cyclin E. Cancer Res. 2011;71:3377–3386. doi: 10.1158/0008-5472.CAN-10-4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nanos-Webb A, Jabbour NA, Multani AS, Wingate H, Oumata N, et al. Targeting low molecular weight cyclin E (LMW-E) in breast cancer. Breast Cancer Res Treat. 2011 doi: 10.1007/s10549-011-1638-4. E-pub ahead of print 22 June 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spruck C, Sun D, Fiegl H, Marth C, Mueller-Holzner E, et al. Detection of low molecular weight derivatives of cyclin E1 is a function of cyclin E1 protein levels in breast cancer. Cancer Res. 2006;66:7355–7360. doi: 10.1158/0008-5472.CAN-05-3240. [DOI] [PubMed] [Google Scholar]
- 22.Delk NA, Hunt KK, Keyomarsi K. Altered subcellular localization of tumor-specific cyclin E isoforms affects cyclin-dependent kinase 2 complex formation and proteasomal regulation. Cancer Res. 2009;69:2817–2825. doi: 10.1158/0008-5472.CAN-08-4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jackman M, Kubota Y, den Elzen N, Hagting A, Pines J. Cyclin A- and cyclin E-Cdk complexes shuttle between the nucleus and the cytoplasm. Mol Biol Cell. 2002;13:1030–1045. doi: 10.1091/mbc.01-07-0361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caruso JA, Hunt KK, Keyomarsi K. The neutrophil elastase inhibitor elafin triggers rb-mediated growth arrest and caspase-dependent apoptosis in breast cancer. Cancer Res. 2010;70:7125–7136. doi: 10.1158/0008-5472.CAN-10-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]