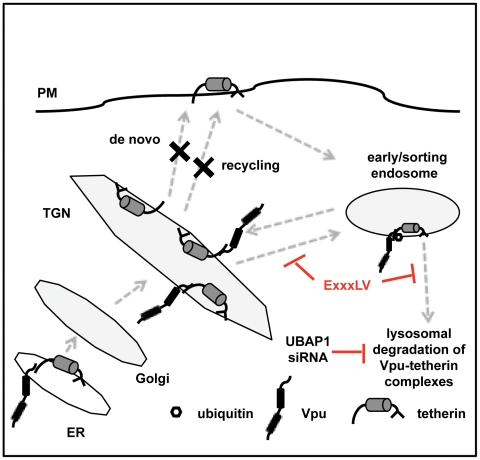
Figure 9. Model for the role of the EXXXLV motif in tetherin antagonism.
Tetherin is expressed at the plasma membrane where it can become incorporated into viral particles or recycles constitutively via early/sorting endosomal compartments and the TGN. Vpu interacts with tetherin in the TGN (and perhaps earlier) through TM-domain-mediated interactions. In the presence of a functional EXXXLV motif, tetherin/Vpu complexes are prevented from trafficking to the PM and routed for ESCRT-dependent endosomal degradation via a clathrin-dependent mechanism. In the absence of an EXXXLV motif, tetherin/Vpu complexes recycle via the PM dependent on the YXYXXV sorting sequence in the tetherin cytoplasmic tail, which interacts with AP-2 and AP-1. During the recycling process, physical interaction of Vpu and/or modification by ubiquitin ligases, such as SCF-β-TrCP2, may further interfere with tetherin function to a variable degree in the absence of cell-surface downregulation.