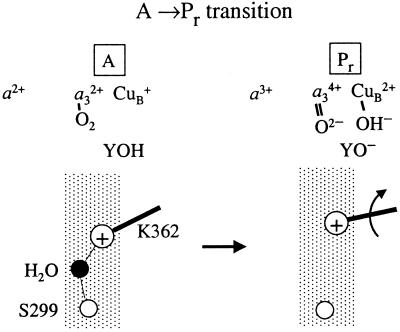
Figure 5.
A schematic model explaining the experimental data in terms of structural events. In the wild-type enzyme, the protonated, charged (+) K(I-362) forms a hydrogen bond with a water molecule, which is hydrogen bonded to S(I-299). Formation of Pr involves the transfer of an additional electron to the binuclear center. To compensate for this negative charge, the K(I-362) side chain has to move toward the binuclear center.