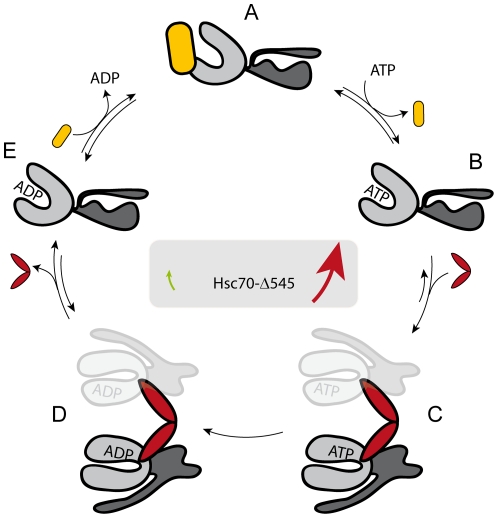
Figure 7. A model for the regulation of CeHsc70's ATPase by the lid domain.
A structural hypothesis for the regulation of the CeHsc70 ATPase cycle may be formulated based on the structures 2KHO of DnaK (74) and 3D2E of the Hsp70-homolog protein Sse1 (75). After initial binding of ATP to the NBD of CeHsc70 (Step A→B), conformational changes result in a hydrolysis competent conformation (Step B→C). This reaction is favored in CeHsc70, as evident from the observation, that hydrolysis is not rate-limiting. The helical lid likely regulates the equilibrium or the kinetics of the B→C transition, as this reaction appears to be much slower in CeHsc70-Δ545. DNJ-13 (red) accelerates the formation of the hydrolysis-competent conformation and thus promotes ATP hydrolysis. ATP hydrolysis likely is irreversible (Step C→D). After hydrolysis, DNJ-13 leaves the complex and Hsc70 returns to its open conformation (Step D→E). BAG-1 (yellow) acts to displace the nucleotide (Step E→A). Based on this model, simultaneous BAG-1 and DNJ-13 binding to CeHsc70 would be mutually exclusive, although several intermediate steps might exist during this sophisticated cycle.