Abstract
Age-related changes in cortical thickness have been observed during adolescence, including thinning in frontal and parietal cortices, and thickening in the lateral temporal lobes. Studies have shown sex differences in hormone-related brain maturation when boys and girls are age-matched, however, because girls mature 1–2 years earlier than boys, these sex differences could be confounded by pubertal maturation. To address puberty effects directly, this study assessed sex differences in testosterone-related cortical maturation by studying 85 boys and girls in a narrow age range and matched on sexual maturity. We expected that testosterone-by-sex interactions on cortical thickness would be observed in brain regions known from the animal literature to be high in androgen receptors. We found sex differences in associations between circulating testosterone and thickness in left inferior parietal lobule, middle temporal gyrus, calcarine sulcus, and right lingual gyrus, all regions known to be high in androgen receptors. Visual areas increased with testosterone in boys, but decreased in girls. All other regions were more impacted by testosterone levels in girls than boys. The regional pattern of sex-by-testosterone interactions may have implications for understanding sex differences in behavior and adolescent-onset neuropsychiatric disorders.
Introduction
Adolescence is a time of dramatic physical, emotional and social change [1] and is also associated with a broad range of problems with behavior and emotion, including sharply increasing rates of depression, suicide, and substance abuse [2]. There is growing interest in achieving a deeper understanding of the normative maturational changes in neural systems that underpin emotion, behavior, and decision-making, in ways that could provide insights into early intervention strategies. One area of interest is focused on understanding puberty-specific maturational changes including the emergence of sex differences (for example the increase in depression in adolescence has been associated directly with the onset of puberty and occurs 2 to 3 times more frequently in girls than boys) [2]. There is evidence for a broader range of sex differences, some established during puberty, that are evident in aspects of cognition and in susceptibility to psychopathology [3], [4], [5]. However, relatively little is known about the neurobiology underlying these differences in maturation. The study of typically developing children and adolescents aids understanding in a period of maturation where the onset of developmental disorders, excessive risk-taking and emphasis on immediate reward can result in pathological outcomes in youth and adults. Models of typical brain development would be improved by evaluation of interactions between hormonal and the still-maturing neural systems responsible for social interactions, affective states and cognitive control. While somewhat controversial, there is significant evidence for sex differences in perceptual (i.e. visuo-spatial) [5], affective (i.e. mood and impulsivity) [6], [7], and language abilities [5] during this period, and these may contribute to differences in practical considerations in, for example, addressing psychopathology and education in our developing children.
Puberty, a sequence of events, which occur during adolescence, is initiated by sex steroid hormone signaling, affecting not only physical sexual maturity, but also reorganization of neural networks that impact behavior. Animal studies have shown that the brain's response to both sensory and steroidal stimulation differs in juveniles, adolescents and adults [8]. Gonadal steroid hormone signaling also has direct functional effects on reproductive behavior through mechanisms akin to neurotransmitter signaling [9]. The long-term organizational effects of hormones on the brain early in development and the activational effects of the same hormones during puberty impact complex behavior [10]. For example, juvenile hamsters castrated prior to and administered testosterone treatments after puberty do not generate normal sexual behavior, highlighting the importance of gonadal steroid hormones in modeling brain circuits [10].
Animal studies have also shown that cellular effects of steroid hormone binding on brain structure are numerous. These include sex differences in the time-course of apoptosis (eg – rat V1) [11], neurotransmitter level changes (eg. cingulate, insular, parietal and occipital cortices, the amygdala, and hypothalamus) [12], [13], and the growth of new cells at puberty [14]. Other sex differences in neurobiology include differences in expression of postsynaptic neurotransmitter receptors [15] or patterns of presynaptic inputs [16], [17] which alter dendritic branching [18], [19], dentritic spine density [20], and connectivity [21]. Further, sex differences in concentrations of both androgen receptors and testosterone (TES) levels in prenatal, peripubertal and adult males and females result in differences in brain structure and function. Focusing on puberty-related sexual differentiation, these studies have found striking sex differences in TES-related patterning in occipital (primary sensory cortex/V1), frontal, and limbic regions. For example, male rodents have more pyramidal branches and dendritic arbors in prefrontal neurons [22] in visual cortices [23] and testosterone levels impact cotrico-cortico motor connections [21]. The higher number of neurons in the visual system in late peripubertal male than female rats has been linked to a suppression of apoptosis by higher testosterone levels in male than female rats [11], [24]. While the animal literature may or may not translate to receptor patterns in humans, we use this as a framework to generate a priori hypotheses in the burgeoning field of relationships between pubertal markers and brain structure in typically developing children and adolescents.
Although the cellular etiology of human brain maturational change observed with structural magnetic resonance imaging (sMRI) is not well understood, human studies have been useful in confirming some of these findings on a macro-scale using non-invasive imaging methods such as sMRI. Recent longitudinal studies with large samples of children and adolescents have shown that gray matter maturation has an inverted u-shaped size-by-age trajectory that varies by region in onset, duration, and shape [25]. As with animals, there are also marked brain changes in humans during puberty and hormone binding in humans likely influences brain development differently in boys than girls. Throughout the brain, there is a net age-related loss of gray matter starting at around age 10 and varying depending on which portion of the cortex measurements are taken, with thinning in dorsal frontal and parietal lobes continuing throughout adolescence and young adulthood that is attributed to gross cellular mechanisms like synaptic pruning and myelination [26], [27], [28], [29]. There is thickening in the medial [30], [31], [32] and lateral temporal lobes until about age 30 [29], [33], [34], perhaps indicative of extended plasticity (i.e., delayed synaptic pruning and myelination) in these regions. The age-related developmental trajectories of male and female adolescents are different [25], and modified by androgen-receptor genetic subtypes [35], confirming results from recent studies of sex-differences in hormone/puberty-correlated maturation ([36], [37], [38]. However, sex differences in both the onset of puberty [39], and in the time-courses of age-related maturation [25], [35], raise questions about whether sex differences in hormone-correlated maturation are due to gross phase differences in development or to actual differences in testosterone-related changes in the brain.
Androgen receptors, targeted by some gonadal steroid hormones, and notably by testosterone, are widely distributed in cortical and subcortical structures [40] and in animals (reviewed above) contribute to significant cellular changes in brain structures during puberty that differ between sexes. In a previous report in the same boys and girls studied here, we found sex differences in volumes of the amygdala and hippocampus, which are important for emotional processing and dense in androgen receptors, that were greater in more sexually mature adolescents. Specifically, sex-differences in the volumes of these specific medial temporal lobe structures were more pronounced in later stages of puberty, where increases in volume were observed in boys, but not girls. We also saw global measures of cortical gray matter volume reductions in later stages of puberty were more prominent in girls than boys [38]. Because frontal lobes are important for impulse control and risk/reward assessments, are known to functionally mature during puberty [41], and have important functional connections with medial temporal lobe structures, such as the amygdala (for review, see [42]), we expected to extend our findings to this report where we focus on cortical thickness.
Using T1-weighted sMRI of 85 adolescent girls and boys matched for pubertal status, assessed using Tanner staging (TS) [43], here, we study sex differences in the relationship between TES levels and cortical gray matter thickness. We did not expect to detect sex differences in cortical thickness, independent of age, in our pubertal status matched population per se, given the small differences in thickness found in previous human studies during the brief age span studied here (approximately 11–14 years) [35]. We did, however, expect to find sex differences in TES-related cortical thickness, independent of age, in regions with high densities of androgen receptors, or high degrees of connectivity with regions expressing high densities of androgen receptors, such as the frontal, limbic, and occipital cortices. Androgen receptors are especially dense within the occipital lobe, limbic cortex (e.g. anterior cingulate gyrus) [44], as well as in numerous non-cortical structures whose projections might exert measurable changes in cortical gray matter thickness [45], [46].
Results
Relationships between age and circulating testosterone levels
We tested whether, even within the restricted age range studied, age was associated with TES level. TES levels were positively correlated with age in boys (r = 0.51, p<0.001) but not girls (r = 0.14, p = 0.34).
Relationship between sex and pubertal status
We tested whether there were differences in pubertal status between participating boys and girls. There was no significant difference between participating boys and girls in Tanner's Stage (p = 0.30).
Sex Differences
Sex differences in cortical thickness and whole brain volume
As expected, we observed no significant differences in our voxel-wise analysis of cortical thickness between boys and girls, independent of age, after correcting for multiple comparisons using the False Discovery Rate (FDR) [47]. While boys had significantly larger (p<0.001) brain volumes than girls (BOYS: mean = 1144518 mm3, SD = 112011; GIRLS: mean = 1032897 mm3, SD = 79081), mean whole brain cortical thickness was not significantly different (p = 0.28) in participating girls (mean = 2.70 mm, SD = 0.08) and boys (mean = 2.68, SD = 0.09).
Sex differences in the effects of TES on cortical thickness, independent of age
To assess whether sex differences in areas with high densities of TES receptors and regions known to be sexually dimorphic during adolescence or adulthood were driven by TES-related change during puberty, we tested for interactions between sex and the effects of TES on gray matter thickness, independent of age using FreeSurfer (http://surfer.nmr.mgh.harvard.edu). We predicted either a less extensive thinning, or even increases in cortical thickness would be associated with increased levels of TES in boys. In girls, conversely, we predicted that more pronounced cortical thinning would generally be associated with increased TES, given findings in longitudinal studies showing a delay in brain maturation (e.g. thinning of frontal cortices) in boys relative to girls [25], [35], and given our previous results of global gray matter volume reductions being more prominent in girls than boys [48].
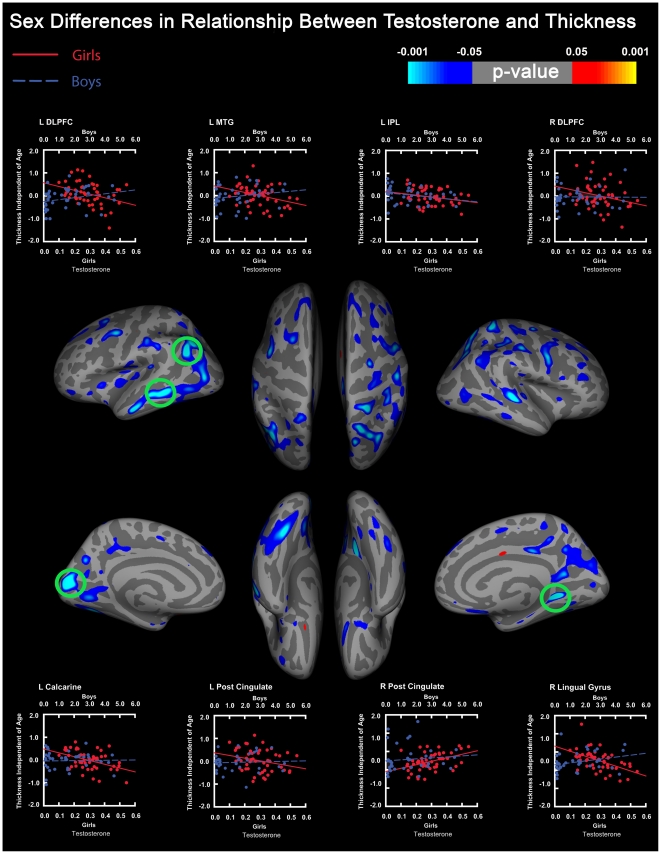
As predicted, we found sex-by-TES interactions predicting gray matter thickness throughout cortical areas known to be especially dense in ARs, including frontal, limbic, and occipital-visual areas. Regions both specifically predicted and significant, after correcting for multiple comparisons using FDR, were in the occipital lobe (left pericalcarine sulcus; the location of primary visual cortex (V1) and higher order visual regions such as the left cuneus and bilateral lingual gyrus). Interactions were driven by the opposite relationship between TES and gray matter thickness in boys and girls in this occipital lobe region (see Fig. 1). Specifically, higher TES levels were associated with thicker cortex in boys but thinner cortex in girls (see Fig. 2).
Figure 1. Results from Sex x TES-Thickness interaction.
Left side of the figure contains results from the left hemisphere. Green circles indicate regions that survived correction for multiple comparisons using false discovery rate (FDR). Scatterplots represent results extracted from center of significant portion in region indicated. Boys are plotted in blue. Girls are plotted in red. Regions plotted include the left dorsal lateral prefrontal cortex (L DLPFC), middle temporal gyrus (L MTG), inferior parietal lobule (L IPL), calcarine sulcus (L Calcarine), posterior cingulate gyrus (L Post Cingulate), right dorsal lateral prefrontal cortex (R DLPFC), posterior cingulate gyrus (R Post Cingulate), and lingual gyrus (R Lingual Gyrus). Regions surviving FDR include the left inferior parietal lobule, middle temporal gyrus, calcarine sulcus, and right lingual gyrus.
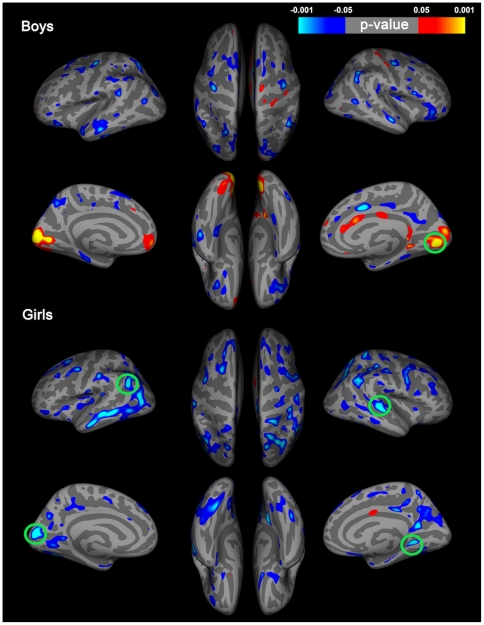
Figure 2. Results from correlating TES levels with thickness, independent of age in boys and girls.
Top of figure shows results of analysis in boys. Bottom of figure shows results of analysis in girls. Left side of figure shows results from the left hemisphere. Green circles indicate regions that survive correction for multiple comparisons using false discovery rate (FDR). Regions surviving FDR correction in boys include the right lingual gyrus. Regions surviving FDR correction in girls include the left inferior parietal lobule, calcarine sulcus, right middle temporal gyrus and lingual gyrus.
We also found sex-by-TES interactions predicting gray matter thickness in the following regions: the bilateral superior and middle frontal gyri, precentral sulcus, posterior cingulate gyrus, lateral orbito-frontal gyrus, left inferior frontal gyrus (including the left pars orbitalis, part of Broca's area), and right medial orbito-frontal gyrus. Here, interactions were driven by multiple mechanisms (see Fig. 1), though generally we found more pronounced TES-related thinning in girls than boys in most regions. In some other regions, interactions were driven by TES related increases in thickness in boys, but not girls (see Fig. 2). While these findings are consistent with our a priori predictions, they did not survive correction for multiple comparisons, and should be interpreted with caution relative to the findings in the occipital lobes.
We also found unpredicted but significant sex-by-TES interactions predicting gray matter thickness, after FDR correction, in the left inferior parietal lobule and left middle temporal gyrus (Fig. 1). Cortical thickness in both girls and boys declined with TES, independent of age, in a similar manner, but the correlation between TES and thickness was significant (after FDR correction) in girls but not boys (Figs. 1 & 2). In the left middle temporal gyrus, TES was associated with cortical thinning in girls, but not boys (Fig. 1).
In addition to the predicted sex-by-TES interactions in occipital cortices, we found unpredicted but significant interactions in other primary sensory areas including the pre- and post- central gyri, locations of primary somatosensory (S1) and motor (M1) regions. The transverse temporal gyrus, the location of primary auditory (A1) cortex showed a trend sex-by-TES-thickness interactions predicting gray matter thickness (α = p<0.05, uncorrected) (Fig. 1). Thickness in the medial occipital cortex (location of primary visual cortex, or V1) and S1 was positively associated with TES levels in boys and negatively associated with TES levels in girls. Thickness in portions of M1 was either not associated, or positively associated with TES levels in boys, but negatively associated with TES levels in girls. A1 appears to be more impacted by TES levels in girls than boys, but in both genders was negatively associated with TES-levels (see scatter plots in Fig. 2).
Boys
Effect of testosterone on cortical thickness, independent of age in boys
We found a positive, significant correlation between TES and the posterior occipital cortex in boys (specifically the right lingual gyrus), corrected for multiple comparisons using FDR (Fig. 2). Other relationships between TES and cortical thickness were observed throughout occipital, limbic and frontal areas, though these regions did not survive correction for multiple comparisons (Fig. 2).
Girls
Effect of testosterone on cortical thickness, independent of age in girls
TES levels were inversely associated with thickness in the occipital lobe (specifically the left calcarine sulcus and right lingual gyrus), independent of age, after correcting for multiple comparisons using FDR (Fig. 2), consistent with our predictions. We also found significant, though not specifically predicted portions of the right superior temporal gyrus to be negatively associated with TES levels after correcting for multiple comparisons using FDR (Fig. 2). Other relationships were found between TES and widespread, bilateral thinning in the posterior cingulate gyrus, entorhinal cortex, precuneus, right parahippocampal gyrus, frontal lobes, including the superior, middle, inferior (including Broca's area) and lateral orbito-frontal gyri, as well as the precentral gyrus of girls, though, these regions did not survive correction for multiple comparisons.
Discussion
Results from our study are the first to indicate that pubertal hormones impact cortical thickness maturation differently in boys than girls. The current study was unique in using a design in which age and physical sexual-maturity differences are controlled, allowing for the examination of the effects of pubertal maturation independent of age in cortical gray matter thickness. The regional patterns of sex differences in testosterone-related cortical thickening and thinning observed here make sense in light of the animal literature and our own findings [49] that there are sex differences in puberty-related patterning within structures dense in, or highly connected to structures dense in ARs [11], [38], [44], [46], [50]. In general, our results demonstrated that limbic and primary sensory cortices of the occipital lobes increased in thickness with increased testosterone in boys at the same time that testosterone-related decreases in thickness were observed in these regions in girls matched to boys for sexual maturity. Conversely, cortical thinning in frontal cortices appeared more accelerated in girls than boys as testosterone levels increased, though, we were unable to test this hypothesis explicitly given the cross-sectional data.
While we did not predict or detect sex differences in gray matter thickness per se in our sexual maturity-, rather than age-matched population of boys and girls, we did anticipate sex differences in the pattern of development of gray matter thickness during pubertal maturation. That is, we observed sex differences in TES-related development of gray matter in our cross sectional sample of adolescents, specifically in cortical regions known to be dense in ARs, like frontal, limbic, and occipital visual areas. To determine what drove sex differences in the association between TES and gray matter, we also tested relationships between TES and gray matter thickness, independent of age, in boys and girls separately. Based on the animal literature, where TES has been found to promote cellular mechanisms that either prevent tissue loss or promote tissue increases at higher levels [11], [23], [51], we expected either increases, or less steep decreases in boys than girls with increasing TES in regions found to be sexually dimorphic in animal models. These hypotheses were supported in our findings of significant sex differences in the TES-related maturational trajectory of visual areas, including early visual areas, specialized in processing the earliest stages of visual processing (e.g. pericalcarine sulcus), and later visual areas (e.g. lingual gyrus and cuneus). Higher TES levels were associated with thinner gray matter in these areas in girls, but with thicker gray matter in boys.
It is important to note that a positive or negative correlation between TES levels and thickness may not represent an effect of TES on thickening or thinning per se. For example, a positive correlation between TES levels and thickness in the visual cortex of boys may represent a preservation of tissue by blocking apoptosis, rather than by stimulating neurogenesis. Indeed, the rodent literature shows that multiple mechanisms are involved in establishing sex differences in the occipital lobe. First, higher TES levels in male than female rats cause an about 20% difference in the number of cells that survive apoptosis [24], leading adult male to have more occipital neurons than adult female rats [11], [23], [51]. There are also differences in synaptic density present in postnatal development, as well as differences in the temporal development of synapses in male and female rats. In early postnatal development, female rats have a higher number of synapses in visual cortex than do male rats, but in later postnatal development, female rats have a decrease in synapse number, while there is evidence that TES spares or even increases these in male rats so that synaptic density becomes similar in fully mature male and female rodents [20].
In addition to predicting sex differences in TES-related patterning in the occipital lobe, we also predicted and found sex differences in frontal cortices including bilateral superior and middle frontal gyri, left inferior frontal gyrus (including the left pars orbitalis, part of Broca's area), bilateral precentral sulcus, and limbic structures (bilateral posterior cingulate gyrus, bilateral lateral orbito-frontal gyrus, right medial orbito-frontal gyrus), though these did not survive correction for multiple comparisons. In general, we found more pronounced TES-related thinning in the frontal regions of girls than boys. These differences may relate to sex differences in adolescent cognition and behavior. For example, differences in puberty related patterning in Broca's area may relate to findings that adolescent girls on average out perform boys in some language tasks [5], or explain why these differences disappear in adulthood (for review, see [52]). Similarly, the less prominent or delayed puberty-related frontal lobe development in boys may explain sex differences in the developmental trajectories found in adolescents, such as the initial delay and subsequent acceleration in male frontal lobe thinning relative to females [27], [35], which has been proposed as an explanation of the peripubertal male bias in accidents, violence, and psychopathology such as drug abuse and suicide [35]. While these (uncorrected) findings should be interpreted with more caution, they may be useful in generating further hypotheses for future studies.
Though unpredicted at the outset of this study, we also found significant (with correction for multiple comparisons using FDR) sex differences in TES-related patterning in the left inferior parietal lobe and left middle temporal gyrus. Our findings may be consistent with recent reports of sex differences in longitudinal developmental trajectories as well as evidence that variability in the maturation of these structures is accounted for by AR subtype. More efficient TES binding was associated with more “masculine” patterning in this region in a previous report [35]. Further, longitudinal studies have shown that during puberty, the temporal lobes mature/thicken more rapidly in boys than girls [35]. Here we show additional evidence that these findings could be rooted in sex differences in TES-related cortical patterning.
Interestingly, we found that TES-levels were inversely associated with thickness in the lateral temporal lobe in girls (corrected for multiple comparisons using FDR), though this region is known to thicken with age during adolescence [33], suggesting that TES is on average suppressing mechanisms that cause apparent thickening on MRI. This is not consistent with findings, specifically in girls, that more efficient TES binding, based on AR receptor subtype, is associated with more rapid thickening [35]. It is possible that a longitudinal study could shed light on these complex interactions. It is also possible that differences in TES levels, age, or AR receptor subtype composition between our population of girls and participants in other studies explain this discrepancy.
In contrast to animal studies that can experimentally control TES levels, this is a study of typically developing youth, which must rely on correlations between TES and thickness. While correlation is not causality, we believe our findings in conjunction with evidence from experimental studies in the animal literature support the hypothesis that changes in TES levels directly impact changes in human brain structure. Given the cross-sectional design of our study, future longitudinal studies are needed to better disentangle how age, time, and TES levels (and perhaps specific experiences) modulate changing brain structure. While our measures of testosterone were collected in the morning, variations in testosterone collected at different times in the day should be the subject of future study. The current study represents an important contribution to the literature on adolescent brain development providing new insights into the importance and specificity of sex-specific changes in neurodevelopment during pubertal maturation. Given the broad range of behavioral and emotional health problems that emerge at this point in development—including sex differences in depression, substance use and some risk-taking behavior, this work highlights the need for further investigations to further delineate sex and puberty specific changes during this dynamic and important period of brain development.
Materials and Methods
Subjects
130 healthy adolescents from Pittsburgh, PA and the surrounding community were recruited through advertisements, flyers, and demographically targeted phone lists. Adolescents were free of lifetime psychiatric disorders, did not have braces, and had no history of head injury, serious medical illness, or psychotropic medication. The University of Pittsburg Institutional Review Board approved human participants research. Written informed consent was obtained from a parent or legal guardian, verbal assent was obtained from the adolescent participants, and all clinical investigation was conducted according to the principles expressed in the Declaration of Helsinki. Twenty-nine participants (22%) were of African-American, two (1.5%) Asian, and two Native-American (1.5%) decent. The remaining participants were Caucasian (75%). Three participants withdrew, seven were excluded due to inadequate image quality, and twenty-eight participants were excluded because we were unable to assay circulating TES levels. A subset of the same subjects described in this report have been evaluated to assess relationships between pubertal maturation and brain activation [53] and to assess relationships between pubertal development and structures in the medial temporal lobes (MTL) [38]. In this manuscript, we do not re-evaluate changes in the MTL. While most participants are used in both studies, some had to be excluded due to issues related to image quality in the MTL, which is more susceptible to artifacts. Table 1 provides demographic descriptions of the 85 participants.
Table 1. Demographic characteristics of 85 normally developing adolescents, by sex.
| Demographics | |||
| Sex | Description | Mean | SD |
| Girls | Age | 11.93 | 0.64 |
| Age Range | 10.75–13.48 | -- | |
| Number (N) | 49 | -- | |
| Mean TS | 3.40 | 1.63 | |
| TS Range | 2–5 | -- | |
| Mean Testosterone | 0.29 | 0.11 | |
| Testosterone Range | 0.10–0.54 | -- | |
| Handedness (Number Right) | 45 | -- | |
| Boys | Age | 12.88 | 0.67 |
| Age Range | 11.73–13.99 | -- | |
| Number (N) | 36 | -- | |
| Mean TS | 3.92 | 2.37 | |
| TS Range | 1–4 | -- | |
| Mean Testosterone | 1.52 | 1.37 | |
| Testosterone Range | 0.12–5.43 | -- | |
| Handedness (Number Right) | 33 | -- | |
Demographics from 85 participating adolescent boys and girls. Demographics are tabulated for girls (TOP) and boys (MIDDLE) as data from each sex was analyzed separately in some statistical tests. Sex differences (BOTTOM) in key demographics of participating boys and girls are tabulated. A one-tailed, two-independent sample t-test was used to calculate sex differences in TS. Two-tailed, two-independent sample t-tests were used to calculate sex differences in age and circulating testosterone.
denotes significance (p<0.05).
Sexual maturity
Participants underwent a physical examination by a research-trained nurse practitioner to determine stage of sexual maturation with the criteria specified by Marshall and Tanner [43]. Group descriptions are detailed in Table 1. Boys and girls were matched for TS using group means.
Circulating testosterone levels
Blood samples were collected and analyzed for TES level in both boys and girls. Samples were obtained at the same time for all subjects (between 8:20 and 8:35 AM), using minimally invasive finger-stick procedure developed by Worthman and colleagues [54]. This method provides several advantages over salivary assays for gonadal steroids, and the correspondence of bloodspot-derived level and plasma level is high. Hormone assays were a modification of a commercially available serum/plasma radioimmunoassay kit (T: DSL/Beckman Coulter, Miami FL; E2 Siemens, Los Angeles, CA). Sensitivity measured as the minimum detectable dose (MDD) and inter-assay coefficients of variation (CV) for low, medium and high BioRad external controls for TES were MDD = .04 ng/mL; CV = 7.2% (low), 11.4% (medium) and 4.3% (high). This assay technique is sensitive enough to detect testosterone levels in girls, who have much lower levels than boys.
Structural image acquisition
All subjects were scanned in a Siemens Allegra head-only 3-Tesla magnet with a 3D T1-weighted protocol (Siemens, Malvern, PA). Scan parameters were as follows: repetition time (TR), 1540 ms; echo time (TE), 3.04 ms, flip angle, 8°; field of view (FOV) 256×256; image voxel size, 1×1×1 mm; acquisition time, 4.48 m.
Image processing
Preprocessing on structural images were conducted in the UCLA Laboratory of Neuro Imaging (LONI) Pipeline Processing Environment [55], [56] and using FreeSurfer's automated segmentation software (FreeSurfer 4.0.3, http://surfer.nmr.mgh.harvard.edu), as described in the work of [57], [58], [59]. During preprocessing, T1-weighted images for each participant were motion corrected using a hybrid watershed/surface deformation procedure [60], brain extracted, intensity normalization [61] tessellation of the gray matter white matter boundary, automated topology correction [62], [63], and surface deformation following intensity gradients to optimally place the gray/white and gray/cerebrospinal fluid borders at the location where the greatest shift in intensity defines the transition to the other tissue class [58], [64].
Additional data processing and analysis was conducted outside of the LONI Pipeline environments, including surface inflation [57], registration to a spherical atlas, which utilized individual cortical folding patterns to match cortical geometry across subjects [65]. This method uses both intensity and continuity information from the entire three dimensional MR volume in segmentation and deformation procedures to produce representations of cortical thickness, calculated as the closest distance from the gray/white boundary to the gray/CSF boundary at each vertex on the tessellated surface [64]. The maps were created using spatial intensity gradients across tissue classes and are therefore not simply reliant on absolute signal intensity. The maps produced are not restricted to the voxel resolution of the original data and thus are capable of detecting submillimeter differences between groups. Procedures for the measurement of cortical thickness have been validated against histological analysis [66] and manual measurements [67], [68]. Freesurfer morphometric procedures have been demonstrated to show good test-retest reliability across scanner manufacturers and across field strengths [69]. It should be noted, that while we studied the volumes of the amygdala and hippocampus in our previous report [38], as both are treated as 3-dimensional volumetric shapes, rather than as part of the 2-dimensional cortical ribbon, and we did not investigate those regions in this analysis of cortical thickness.
Statistical Analyses
We used a one-tailed two-independent sample t-test [70] to test whether participating boys had larger whole brain volumes than participating girls. Sex differences in whole brain thickness were evaluated using a two-tailed two-independent sample t-test [71]. Mean thickness was calculated using regional measures extracted using Freesurfer tools and by averaging thickness in each region in both hemispheres. We also used a two-tailed two-independent sample t-test to test whether participating boys and girls were in significantly different stages of puberty, based on Tanner's Stage.
All other analyses were conducted within the FreeSurfer statistical software package using an alpha of p<0.05 (both voxel-wise and when correcting for multiple comparisons using FDR) and simultaneous multiple regression analysis. To assess SEX-by-TES-thickness interactions, independent of age, we used the following linear model: THICKNESS = CONSTANT+beta1SEX+beta2TES+beta3SEX+TES beta4AGE+ERROR. To understand the underlying effects of TES on thickness in boys and girls separately, independent of age, we used the following linear model: THICKNESS = CONSTANT+beta1TES+beta2AGE+ERROR.
Acknowledgments
We would like to thank Eric Kan for managing the data, Cornelius Hojatkashani and the rest of the LONI Pipeline Team, Rico Magsipoc and Jonathan Pierce for improving and maintaining our computing resources.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by the National Institute of Drug Abuse Grants R01 DA017831 awarded to ERS and R01 DA018910 awarded to RED. Support for this research was also provided by the National Institute of Mental Health Grants R01 MH087563 and National Institute of Child Development Health and Human Development RO1 HD053893-01 awarded to ERS and the Undergraduate Research Scholars grant to JAH, supported by the Hilton Family Foundation. Additional support was provided by the National Institutes of Health through the National Institute of Health Roadmap for Medical Research, grant U54 RR021813 entitled Center for Computational Biology and NIH/NCRR 5 P41 RR013642 awarded to AWT. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Yurgelun-Todd D. Emotional and cognitive changes during adolescence. Curr Opin Neurobiol. 2007;17:251–257. doi: 10.1016/j.conb.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 2.Dahl RE, Gunnar MR. Heightened stress responsiveness and emotional reactivity during pubertal maturation: implications for psychopathology. Dev Psychopathol. 2009;21:1–6. doi: 10.1017/S0954579409000017. [DOI] [PubMed] [Google Scholar]
- 3.Gaub M, Carlson CL. Gender differences in ADHD: a meta-analysis and critical review. J Am Acad Child Adolesc Psychiatry. 1997;36:1036–1045. doi: 10.1097/00004583-199708000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Weissman MM, Bland R, Joyce PR, Newman S, Wells JE, et al. Sex differences in rates of depression: cross-national perspectives. J Affect Disord. 1993;29:77–84. doi: 10.1016/0165-0327(93)90025-f. [DOI] [PubMed] [Google Scholar]
- 5.Halpern DF, Tan U. Stereotypes and steroids: using a psychobiosocial model to understand cognitive sex differences. Brain Cogn. 2001;45:392–414. doi: 10.1006/brcg.2001.1287. [DOI] [PubMed] [Google Scholar]
- 6.Cross CP, Copping LT, Campbell A. Sex differences in impulsivity: a meta-analysis. Psychol Bull. 2011;137:97–130. doi: 10.1037/a0021591. [DOI] [PubMed] [Google Scholar]
- 7.Hankin BL, Mermelstein R, Roesch L. Sex differences in adolescent depression: stress exposure and reactivity models. Child Dev. 2007;78:279–295. doi: 10.1111/j.1467-8624.2007.00997.x. [DOI] [PubMed] [Google Scholar]
- 8.Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nat Neurosci. 2004;7:1040–1047. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- 9.Remage-Healey L, Maidment NT, Schlinger BA. Forebrain steroid levels fluctuate rapidly during social interactions. Nat Neurosci. 2008;11:1327–1334. doi: 10.1038/nn.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schulz KM, Richardson HN, Zehr JL, Osetek AJ, Menard TA, et al. Gonadal hormones masculinize and defeminize reproductive behaviors during puberty in the male Syrian hamster. Horm Behav. 2004;45:242–249. doi: 10.1016/j.yhbeh.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 11.Nunez JL, Jurgens HA, Juraska JM. Androgens reduce cell death in the developing rat visual cortex. Brain Res Dev Brain Res. 2000;125:83–88. doi: 10.1016/s0165-3806(00)00126-7. [DOI] [PubMed] [Google Scholar]
- 12.Siddiqui A, Shah BH. Neonatal androgen manipulation differentially affects the development of monoamine systems in rat cerebral cortex, amygdala and hypothalamus. Brain Res Dev Brain Res. 1997;98:247–252. doi: 10.1016/s0165-3806(96)00171-x. [DOI] [PubMed] [Google Scholar]
- 13.Stewart J, Rajabi H. Estradiol derived from testosterone in prenatal life affects the development of catecholamine systems in the frontal cortex in the male rat. Brain Res. 1994;646:157–160. doi: 10.1016/0006-8993(94)90070-1. [DOI] [PubMed] [Google Scholar]
- 14.Ahmed EI, Zehr JL, Schulz KM, Lorenz BH, Doncarlos LL, et al. Pubertal hormones modulate the addition of new cells to sexually dimorphic brain regions. Nat Neurosci. 2008 doi: 10.1038/nn.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang L, Ma W, Barker JL, Rubinow DR. Sex differences in expression of serotonin receptors (subtypes 1A and 2A) in rat brain: a possible role of testosterone. Neuroscience. 1999;94:251–259. doi: 10.1016/s0306-4522(99)00234-1. [DOI] [PubMed] [Google Scholar]
- 16.Kritzer MF, Adler A, Marotta J, Smirlis T. Regionally selective effects of gonadectomy on cortical catecholamine innervation in adult male rats are most disruptive to afferents in prefrontal cortex. Cereb Cortex. 1999;9:507–518. doi: 10.1093/cercor/9.5.507. [DOI] [PubMed] [Google Scholar]
- 17.King JA, Barkley RA, Delville Y, Ferris CF. Early androgen treatment decreases cognitive function and catecholamine innervation in an animal model of ADHD. Behav Brain Res. 2000;107:35–43. doi: 10.1016/s0166-4328(99)00113-8. [DOI] [PubMed] [Google Scholar]
- 18.Stewart J, Kolb B. Dendritic branching in cortical pyramidal cells in response to ovariectomy in adult female rats: suppression by neonatal exposure to testosterone. Brain Res. 1994;654:149–154. doi: 10.1016/0006-8993(94)91581-4. [DOI] [PubMed] [Google Scholar]
- 19.Kolb B, Stewart J. Sex-related differences in dendritic branching of cells in the prefrontal cortex of rats. J Neuroendocrinol. 1991;3:95–99. doi: 10.1111/j.1365-2826.1991.tb00245.x. [DOI] [PubMed] [Google Scholar]
- 20.Munoz-Cueto JA, Garcia-Segura LM, Ruiz-Marcos A. Developmental sex differences and effect of ovariectomy on the number of cortical pyramidal cell dendritic spines. Brain Res. 1990;515:64–68. doi: 10.1016/0006-8993(90)90577-x. [DOI] [PubMed] [Google Scholar]
- 21.Venkatesan C, Kritzer MF. Perinatal gonadectomy affects corticocortical connections in motor but not visual cortex in adult male rats. J Comp Neurol. 1999;415:240–265. [PubMed] [Google Scholar]
- 22.Markham JA, Juraska JM. Aging and sex influence the anatomy of the rat anterior cingulate cortex. Neurobiol Aging. 2002;23:579–588. doi: 10.1016/s0197-4580(02)00004-0. [DOI] [PubMed] [Google Scholar]
- 23.Reid SN, Juraska JM. Sex differences in neuron number in the binocular area of the rat visual cortex. J Comp Neurol. 1992;321:448–455. doi: 10.1002/cne.903210311. [DOI] [PubMed] [Google Scholar]
- 24.Nunez JL, Lauschke DM, Juraska JM. Cell death in the development of the posterior cortex in male and female rats. J Comp Neurol. 2001;436:32–41. [PubMed] [Google Scholar]
- 25.Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, et al. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 2007;36:1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 27.Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, et al. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci. 2008;28:3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, et al. Mapping Cortical Change Across the Human Life Span. Nature Neuroscience. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- 29.Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, et al. Longitudinal Mapping of Cortical Thickness and Brain Growth in Normal Children. Journal of Neuroscience. 2004;24:8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giedd JN, Vaituzis AC, Hamburger SD, Lange N, Rajapakse JC, et al. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4–18 years. J Comp Neurol. 1996;366:223–230. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 31.Sowell ER, Jernigan TL. Further MRI evidence of late brain maturation: Limbic volume increases and changing asymmetries during childhood and adolescence. Developmental Neuropsychology. 1998;14:599–617. [Google Scholar]
- 32.Yurgelun-Todd DA, Killgore WD, Cintron CB. Cognitive correlates of medial temporal lobe development across adolescence: a magnetic resonance imaging study. Percept Mot Skills. 2003;96:3–17. doi: 10.2466/pms.2003.96.1.3. [DOI] [PubMed] [Google Scholar]
- 33.Sowell ER, Thompson PM, Tessner KD, Toga AW. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: Inverse relationships during postadolescent brain maturation. J Neurosci. 2001;21:8819–8829. doi: 10.1523/JNEUROSCI.21-22-08819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sowell ER, Thompson PM, Toga AW. Mapping changes in the human cortex throughout the span of life. Neuroscientist. 2004;10:372–392. doi: 10.1177/1073858404263960. [DOI] [PubMed] [Google Scholar]
- 35.Raznahan A, Lee Y, Stidd R, Long R, Greenstein D, et al. Longitudinally mapping the influence of sex and androgen signaling on the dynamics of human cortical maturation in adolescence. Proc Natl Acad Sci U S A. 2010;107:16988–16993. doi: 10.1073/pnas.1006025107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neufang S, Specht K, Hausmann M, Gunturkun O, Herpertz-Dahlmann B, et al. Sex Differences and the Impact of Steroid Hormones on the Developing Human Brain. Cereb Cortex. 2008 doi: 10.1093/cercor/bhn100. [DOI] [PubMed] [Google Scholar]
- 37.Peper JS, Brouwer RM, Schnack HG, van Baal GC, van Leeuwen M, et al. Sex steroids and brain structure in pubertal boys and girls. Psychoneuroendocrinology. 2009;34:332–342. doi: 10.1016/j.psyneuen.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 38.Bramen JE, Hranilovich JA, Dahl RE, Forbes EE, Chen J, et al. Puberty Influences Medial Temporal Lobe and Cortical Gray Matter Maturation Differently in Boys Than Girls Matched for Sexual Maturity. Cereb Cortex. 2010 doi: 10.1093/cercor/bhq137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marshall WA. Puberty. In: Tanner J, Falkner F, editors. Human Growth: A compendium Treatise. 2nd ed. New York: Plenum Press; 1986. pp. 171–209. [Google Scholar]
- 40.Kritzer M. The distribution of immunoreactivity for intracellular androgen receptors in the cerebral cortex of hormonally intact adult male and female rats: localization in pyramidal neurons making corticocortical connections. Cereb Cortex. 2004;14:268–280. doi: 10.1093/cercor/bhg127. [DOI] [PubMed] [Google Scholar]
- 41.Lu L, Leonard C, Thompson P, Kan E, Jolley J, et al. Normal developmental changes in inferior frontal gray matter are associated with improvement in phonological processing: a longitudinal MRI analysis. Cereb Cortex. 2007;17:1092–1099. doi: 10.1093/cercor/bhl019. [DOI] [PubMed] [Google Scholar]
- 42.McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- 43.Marshall WA, Tanner JM. Growth and physiological development during adolescence. Annu Rev Med. 1968;19:283–300. doi: 10.1146/annurev.me.19.020168.001435. [DOI] [PubMed] [Google Scholar]
- 44.Nunez JL, Huppenbauer CB, McAbee MD, Juraska JM, DonCarlos LL. Androgen receptor expression in the developing male and female rat visual and prefrontal cortex. J Neurobiol. 2003;56:293–302. doi: 10.1002/neu.10236. [DOI] [PubMed] [Google Scholar]
- 45.Cooke BM, Woolley CS. Gonadal hormone modulation of dendrites in the mammalian CNS. J Neurobiol. 2005;64:34–46. doi: 10.1002/neu.20143. [DOI] [PubMed] [Google Scholar]
- 46.Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- 47.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society Series B (Methodological) 1995;57:289–300. [Google Scholar]
- 48.Bauer M, London ED, Rasgon N, Berman SM, Frye MA, et al. Supraphysiological doses of levothyroxine alter regional cerebral metabolism and improve mood in bipolar depression. Mol Psychiatry. 2005;10:456–469. doi: 10.1038/sj.mp.4001647. [DOI] [PubMed] [Google Scholar]
- 49.Bramen JE, Hranilovich JA, Dahl RE, Forbes EE, Chen J, et al. Puberty influences medial temporal lobe and cortical gray matter maturation differently in boys than girls matched for sexual maturity. Cereb Cortex. 21:636–646. doi: 10.1093/cercor/bhq137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cooke BM. Steroid-dependent plasticity in the medial amygdala. Neuroscience. 2006;138:997–1005. doi: 10.1016/j.neuroscience.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 51.Nunez JL, Sodhi J, Juraska JM. Ovarian hormones after postnatal day 20 reduce neuron number in the rat primary visual cortex. J Neurobiol. 2002;52:312–321. doi: 10.1002/neu.10092. [DOI] [PubMed] [Google Scholar]
- 52. Halpern DF. 2000. Sex differences in cognitive abilities Mahwah, N.J. L. Erlbaum Associates; xviii, 420 [Google Scholar]
- 53.Forbes EE, Ryan ND, Phillips ML, Manuck SB, Worthman CM, et al. Healthy adolescents' neural response to reward: associations with puberty, positive affect, and depressive symptoms. J Am Acad Child Adolesc Psychiatry. 2010;49:162–172 e161–165. doi: 10.1097/00004583-201002000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Worthman CM, Stallings JF. Hormone measures in finger-prick blood spot samples: new field methods for reproductive endocrinology. Am J Phys Anthropol. 1997;104:1–21. doi: 10.1002/(SICI)1096-8644(199709)104:1<1::AID-AJPA1>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 55.Dinov I, Lozev K, Petrosyan P, Liu Z, Eggert P, et al. Neuroimaging Study Designs, Computational Analyses and Data Provenance Using the LONI Pipeline. PLoS ONE. 2010;5:e13070. doi: 10.1371/journal.pone.0013070. doi: 13010.11371/journal.pone.0013070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rex DE, Ma JQ, Toga AW. The LONI Pipeline Processing Environment. Neuroimage. 2003;19:1033–1048. doi: 10.1016/s1053-8119(03)00185-x. [DOI] [PubMed] [Google Scholar]
- 57.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- 58.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 59.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 60.Segonne F, Dale AM, Busa E, Glessner M, Salat D, et al. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22:1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 61.Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- 62.Fischl B, Liu A, Dale AM. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging. 2001;20:70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- 63.Segonne F, Pacheco J, Fischl B. Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. IEEE Trans Med Imaging. 2007;26:518–529. doi: 10.1109/TMI.2006.887364. [DOI] [PubMed] [Google Scholar]
- 64.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp. 1999;8:272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rosas HD, Liu AK, Hersch S, Glessner M, Ferrante RJ, et al. Regional and progressive thinning of the cortical ribbon in Huntington's disease. Neurology. 2002;58:695–701. doi: 10.1212/wnl.58.5.695. [DOI] [PubMed] [Google Scholar]
- 67.Kuperberg GR, Broome MR, McGuire PK, David AS, Eddy M, et al. Regionally localized thinning of the cerebral cortex in schizophrenia. Arch Gen Psychiatry. 2003;60:878–888. doi: 10.1001/archpsyc.60.9.878. [DOI] [PubMed] [Google Scholar]
- 68.Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, et al. Thinning of the cerebral cortex in aging. Cereb Cortex. 2004;14:721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- 69.Han X, Jovicich J, Salat D, van der Kouwe A, Quinn B, et al. Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. Neuroimage. 2006;32:180–194. doi: 10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- 70.Che A, Cui J, Dinov I. SOCR Analyses: Implementation and Demonstration of a New Graphical Statistics Educational Toolkit. JSS. 2009;30:1–19. doi: 10.18637/jss.v030.i03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Che A, Cui J, Dinov I. SOCR Analyses – an Instructional Java Web-based Statistical Analysis Toolkit. JOLT. 2009;5:1–19. [PMC free article] [PubMed] [Google Scholar]