Abstract
Purpose
Identification of vulnerable plaques remains crucial for better cardiovascular risk assessment. At least 20% of inflammatory cells within unstable (vulnerable) plaques comprise T lymphocytes, which contain receptors for interleukin-2 (IL-2); those receptors can be identified by scintigraphy with radiolabelled IL-2.The aim of this study was to identify the “inflamed” (vulnerable) plaques by scintigraphy using IL-2 labelled with 99mTc in the selected, high cardiovascular risk group of end-stage renal disease (ESRD) patients.
Methods
A total of 28 patients (18 men, 10 women, aged 55.2 ± 9.6 years, 17 on peritoneal dialysis, 11 on haemodialysis) underwent common carotid artery (CCA) scintigraphy with the use of 99mTc-hydrazinonicotinamide (HYNIC)-IL-2. In all cases, ultrasound examination of the CCA was performed and levels of selected proinflammatory factors, atherogenic markers and calcium-phosphate balance parameters were measured. Finally, the target to non-target (T/nT) ratio of IL-2 uptake in atherosclerotic plaques with intima-media thickness (IMT), classic cardiovascular risk factors and concentrations of the measured factors were compared.
Results
Increased 99mTc-HYNIC-IL-2 uptake in atherosclerotic plaques in 38/41 (91%) cases was detected. The median T/nT ratio of focal 99mTc-HYNIC-IL-2 uptake in atherosclerotic plaques was 2.35 (range 1.23–3.63). The mean IMT value on the side of plaques assessed by scintigraphy was 0.79 ± 0.18 mm (median 0.8, range 0.5–1.275).
Correlations between T/nT ratio and homocysteine (R = 0.22, p = 0.037), apolipoprotein B (apoB) (R = 0.31, p = 0.008), apoB to apoA-I ratio (R = 0.29, p = 0.012) and triglyceride concentration (R = 0.26, p = 0.021) were detected. A lower T/nT ratio in patients with better parameters of nutritional status (haemoglobin, albumin, adiponectin) in comparison with patients with worse nutritional parameters (3.20 ± 0.5 vs 2.16 ± 0.68, p = 0.025) was revealed as well as a difference between values of T/nT ratio in groups of patients with values of apoB, soluble CD40 ligand and asymmetric dimethylarginine above and below median (3.18 ± 0.52 vs 2.16 ± 0.68, p = 0.031). No statistically significant association was found between T/nT ratio and mean value of either IMT or classic cardiovascular risk factors.
Conclusion
Scintigraphy with the use of 99mTc-HYNIC-IL-2 can be a tool for inflamed atherosclerotic (vulnerable) plaque visualization within CCA in ESRD patients. Quantitative results of carotid artery scintigraphy with 99mTc-HYNIC-IL-2 correlate with serum concentration of selected cardiovascular risk markers.
Keywords: 99mTc-IL-2, Scintigraphy, Vulnerable plaque, Inflammation, End-stage renal disease (ESRD)
Introduction
Routinely used methods for quantification of short-term cardiovascular risk are considered inefficient. Most of the available methods are based on insight into the arterial lumen, namely assessment of atherosclerotic plaque size, its degree of calcification or intima-media complex thickness measurements. These methods may, however, be imprecise in identifying “vulnerable patients” with plaques at the highest risk of rupture. Providing such information would seem to be very important since it may potentially result not only in more sensitive selection of patients for invasive procedures, but also for choosing candidates best suited to aggressive conservative treatment (leading to stabilization of “vulnerable” plaques). This kind of information may potentially be obtained with scintigraphy using deliberately chosen tracers. Among a variety of candidates, the most promising substances are those involved in regulation of the inflammatory process.
Interleukin-2 (IL-2) was identified in 1975 as a growth factor for T lymphocytes and is now considered one of the most important modulators of the inflammatory response. IL-2 is produced mainly by the CD4+ lymphocyte subpopulation, and its biological activity is attained after binding to specific receptors located, among other target cells, on these lymphocytes [1]. IL-2 induces autoproliferation of helper T cells and cytotoxic lymphocytes as well as B lymphocytes, NK (natural killer) and memory cells [2]. IL-2 secretion by activated lymphocytes also stimulates other immunomodulating cytokines, such as interferon gamma (IFN-γ) or tumour necrosis factor beta (TNF-β), which in turn interact with cells from the monocyte/macrophage lineage, endothelial layer and fibroblasts leading to fibrous cap thickening and plaque vulnerability.
IL-2 radiolabelled with 99mTc or 123I has previously been proven a useful tracer in imaging of the lymphocytic tissue infiltration in patients with autoimmune disorders [3–5] or lymphocyte infiltration into atherosclerotic plaques in patients with symptomatic atherosclerosis [6, 7].
In certain populations, including patients with end-stage renal disease (ESRD), cardiovascular disease is more common and more accelerated than in the general population. This makes the ESRD group a valuable “model” for advanced and rapidly progressing atherosclerosis, useful for clinical studies. The increased cardiovascular mortality in ESRD patients is based mainly on a chronic inflammatory process (the malnutrition-inflammation-atherosclerosis syndrome) as well as chronic anaemia, bone and mineral disorders, hypertension and uraemia with derangement of the biochemical milieu with accumulation of several cardiotoxic and atherogenic substances. Most of these abnormalities mentioned are poorly corrected with dialysis [8].
Serum concentrations of a wide range of inflammatory agents that promote atherosclerosis or indicate severity of endothelial impairment are used to evaluate the cardiovascular risk in ESRD patients [9, 10] as well as in the general population [11–14]. Simultaneous assessments of several agents enable better cardiovascular risk estimation because none of them is sufficient as a single risk indicator.
The aim of the study was to identify the “inflamed” (vulnerable) atherosclerotic plaques within common carotid arteries (CCA) using 99mTc-labelled IL-2 scintigraphy in a selected group of ESRD patients who are at high cardiovascular risk.
Materials and methods
A total of 28 patients (10 women, 18 men, aged 55.2 ± 9.6 years) were included in the study. All patients were treated with dialysis (17 on peritoneal dialysis, 11 on haemodialysis) due to ESRD for a median period of 38.4 months (range 4–133 months). Fifteen patients had diagnosed angina pectoris in a stable state (including three patients with a history of myocardial infarction). Information about other “classic” cardiovascular risk factors (age, sex, diabetes mellitus, hypertension, hypercholesterolaemia, hyperhomocysteinaemia, smoking) was obtained based on patient histories. The body mass index (BMI) was calculated on the day of scintigraphy. Additionally, information about receiving drugs including statins, angiotensin-converting enzyme inhibitors (ACEI) and angiotensin II receptor antagonists was collected.
The main inclusion criterion was a stable clinical status over a period of at least 2 months prior to assessment (i.e. displaying no symptoms of acute coronary event, neoplasm, infectious or non-infectious inflammatory disease, including dialysis-related peritonitis or vascular access infection). None of the patients suffered from a systemic inflammatory disease or received steroids, non-steroidal anti-inflammatory drugs, antibiotics or immunosuppressive treatment.
In addition to scintigraphy, an ultrasound examination of the carotid arteries was performed in order to assess the amount and localization of atherosclerotic plaques and to measure CCA intima-media thickness (IMT).
Furthermore, serum or plasma levels of selected “traditional” and novel markers of inflammation and atherosclerosis were measured:
Markers of inflammation:
C-reactive protein (CRP) (immunonephelometric method, N high sensitivity CRP, Dade Behring GmbH, Marburg, Germany)
Fibrinogen (immunonephelometric method, Multifibren U reagent, Dade Behring GmbH, Marburg, Germany)
Interleukin-6 (IL-6) [enzyme-linked immunosorbent assay (ELISA); Quantikine HS, human IL-6, R&D Systems, Minneapolis, MN, USA]
Interleukin-18 (IL-18) (ELISA; Medical & Biological Laboratories Co., Naka-Ku Nagoya, Japan)
Plasma soluble IL-2 receptor (sIL-2R) (ELISA; Quantikine, human IL-2 sRalpha, R&D Systems, Minneapolis, MN, USA)
Human amyloid A (SAA) (immunonephelometric method, SAA, Dade Behring GmbH, Marburg, Germany)
Homocysteine (Hcy) [enzyme immunosorbent assay (EIA), homocysteine test, Bio-Rad Laboratories, Hercules, CA, USA]
Soluble intercellular adhesion molecule (sICAM) (EIA; human sICAM-1, R&D Systems, Minneapolis, MN, USA)
Antibodies against heat shock proteins 60 (aHSP60) (ELISA; Anti-Human Hsp60 (total) ELISA Kit, Assay Designs Inc., Ann Arbor, MI, USA)
Soluble CD40 ligand (sCD40L) (ELISA; Human sCD40L ELISA, Diaclone SAS, Besançon, France)
Asymmetric dimethylarginine (ADMA) (ELISA; ADMA direct ELISA Kit, Immundiagnostik AG, Bensheim, Germany)
Calcium-phosphate balance parameters:
Total calcium (Ca) and ionized calcium (Hitachi 917, Roche Diagnostics, Division of Hoffmann La Roche, Basel, Switzerland)
Phosphate (P) (Hitachi 917, Roche Diagnostics, Division of Hoffmann-La Roche, Basel, Switzerland)
Calcium-phosphate index was calculated based on results of calcium and phosphate serum concentration (Ca × P)
Intact parathormone (iPTH) (chemiluminescence method; Immulite 2000, Diagnostic Products Corporation, Los Angeles, CA, USA)
Detailed lipid profile and anti-atherogenic factors:
Total cholesterol (total-C) (Hitachi 917, Roche Diagnostics, Division of Hoffmann-La Roche, Basel, Switzerland)
Low-density lipoprotein (LDL) cholesterol (LDL-C) (Hitachi 917, Roche Diagnostics, Division of Hoffmann-La Roche, Basel, Switzerland)
High-density lipoprotein (HDL) cholesterol (HDL-C) (Hitachi 917, Roche Diagnostics, Division of Hoffmann-La Roche, Basel, Switzerland)
Triglyceride (TG) (Hitachi 917, Roche Diagnostics, Division of Hoffmann-La Roche, Basel, Switzerland)
Apolipoprotein A-I (immunonephelometric method, N Antisera to Apolipoprotein A-I and Apolipoprotein B, Dade Behring GmbH, Marburg, Germany)
Apolipoprotein B (immunonephelometric method, N Antisera to Apolipoprotein A-I and Apolipoprotein B, Dade Behring GmbH, Marburg, Germany)
Adiponectin (ELISA; human adiponectin, R&D Systems, Minneapolis, MN, USA)
Markers of oxidative stress:
Oxidized LDL (oxLDL) (ELISA; oxLDL ELISA Kit, Immundiagnostik AG, Bensheim, Germany)
Antibodies against oxidized LDL (anti-oxLDL) (ELISA; anti-oxLDL IgG OLAB IgG, Biomedica Gruppe, GmbH & Co KG, Vienna, Austria)
All of the above analyses were performed in duplicate. Intra-assay variability of measurements was below 3%.
Ten healthy (without any signs of cardiovascular disease) age- and sex-matched volunteers from among our clinic staff served as a control group for biochemical analysis (for certain ethical reasons we found it inappropriate to perform scintigraphy in control subjects).
Method of IL-2 labelling with 99mTc
The method for 99mTc radiolabelling of recombinant human IL-2 (rhIL-2) (Proleukin, Chiron Corp, Emeryville, CA, USA) via hydrazinonicotinamide (HYNIC) leading to the development of a dry kit has been previously described in detail [15]. In that paper we provided the details on tracer optimization, including the HYNIC to IL-2 molar ratio, the influence of HYNIC-IL-2 conjugate mass on specific activity of obtained 99mTc-HYNIC-IL-2 and effect of co-ligands on stability of 99mTc-HYNIC-IL-2 both in vitro and in vivo. The biological activity of 99mTc-HYNIC-IL-2 in the presence of tricine and nicotinic acid (NA) as co-ligands for 99mTc has been documented in a lymphocyte proliferation assay. These studies resulted in the kit formulation rendering the sterile and ready-to-use 99mTc-HYNIC-IL-2(tricine-NA)-rhIL-2 stable within 1 h after completion of the labelling procedure. Briefly, the conjugates of HYNIC-rhIL-2 were prepared using 2 molar excess of HYNIC-HCl and purified on a Sephadex G-25 column (PD-10, GE) using 0.05 M phosphate buffer (pH 7.2) and 0.05% sodium dodecyl sulphate (SDS) in order to prevent protein aggregation. Dry kits containing 30 μg HYNIC-rhIL-2 conjugate, glucose, phosphate buffer, SDS, NaCl, ascorbic acid, NA and tricine as co-ligands in vial I, and tricine and stannous chloride in vial II, were prepared. For labelling around 1.0 ml of radioactive 99mTc in the form of sodium pertechnetate 800–1,000 MBq obtained from the 99Mo/99mTc radionuclide generator (POLATOM, Poland) was added to vial II. After incubation at room temperature for 20 min, 0.5 ml of the 99mTc-tricine complex solution was transferred to vial I containing HYNIC-rhIL-2 conjugate, followed by incubation at 75°C for 30 min. After purification on a PD-10 column around 99% pure monomer of 99mTc-HYNIC-(NA,tricine)-rhIL-2 was obtained with specific activity greater than 4 GBq/mg of rhIL-2 (62.5 GBq/μmol). Aseptic conditions were maintained during tracer preparation and the final product was checked for sterility and bacterial endotoxins.
CCA plaque scintigraphy
The scintigraphic examinations were performed using a dual-head, large field of view e.cam gamma camera (Siemens) equipped with parallel, low-energy high-resolution (LEHR) collimators connected to the syngo MI molecular imaging system workstation.
Single photon emission computed tomography (SPECT) of the neck was performed 1 h after injection of the tracer. Every time 128 frames (acquisition time 45 ) were performed on a 64×64 matrix. The target to non-target (T/nT) ratio for focal 99mTc-HYNIC-IL-2 uptake was calculated within the largest atherosclerotic plaque previously identified by the CCA ultrasound.
SPECT images were reconstructed using the filtered backprojection (FBP) method of reconstruction using a Hanning filter and an attenuation coefficient of 0.125 cm−1. Eight to ten transaxial sections, each 2 cm thick, were reconstructed from the origin of the common carotid upwards. 99mTc-HYNIC-IL-2 uptake as a region of interest (ROI; target, T) was calculated on SPECT images at the level of the carotid bifurcation in the place depicted by ultrasonography. The background (nT) of 99mTc-HYNIC-IL-2 uptake was counted in the region drawn laterally to the ROI adjacent to the CCA.
CCA ultrasound and IMT measurement
The CCA IMT was assessed in B-mode presentation using GE Vivid 7 equipment with 5/7 MHz linear transducer (GE Healthcare, Chalfont St Giles, UK).
An atherosclerotic plaque was defined as an echostructure with a thickness greater than at least 50% compared to neighbouring sites with protrusion into the vascular lumen. Additionally, IMT was measured as an indicator of atherosclerosis advancement on the far wall of the CCA 2–4 cm proximally from its bifurcation. IMT had been assessed bilaterally, but for the purpose of this analysis the result from the side that displayed the plaque(-s) (or the larger plaque) was used. The CCA ultrasound was performed on the day of the scintigraphy examination about 1 h before injection of the tracer.
Laboratory assessment
Blood for the analyses was drawn on the day of scintigraphy prior to the procedure. Fasting blood samples were taken for analyses of serum concentrations of acute phase reactants, cytokines, adhesion molecules, markers of oxidative stress, calcium-phosphate balance parameters and the extended lipid profile. The blood was centrifuged at a temperature of 22°C at a speed of 3,000 g for 15–30 min and stored at a temperature of −80° until assayed.
The study protocol was reviewed and accepted by the independent Ethics Committee of our University. All patients gave signed informed consent prior to participation in the study.
Statistical analysis
Differences between the studied and control group (biochemical tests) were analysed using the χ2 test and the Fisher exact test (if small numbers occurred) for categorical variables. The Mann-Whitney tests were applied for continuous variables. The Mann-Whitney test was also used to discover factors associated with the scintigraphy result in the studied group. When categorized independent variables had more than two values, the Kruskal-Wallis tests were applied.
The odds ratios of T/nT higher than median (2.2) were calculated using logistic regression. In tested models, independent variables were used as continuous as well as categorized for terciles. The statistical significance was defined as p <0.05. All calculations were made using the Stata 8.0 statistical package.
Results
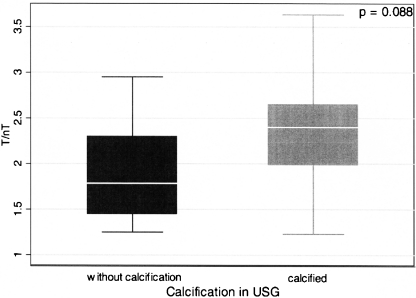
Increased 99mTc-HYNIC-IL-2 uptake was detected in 38 of 41 atherosclerotic plaques (91%) previously visualized by neck ultrasound. The median T/nT ratio of focal 99mTc-HYNIC-IL-2 uptake in the region of atherosclerotic plaques equalled 2.35 (range 1.23–3.63; average 2.35 ± 0.70). The mean IMT value for the CCA with plaque assessed with scintigraphy averaged 0.79 ± 0.18 mm (median 0.8; range 0.5–1.275). No statistically significant association was found between 99mTc-HYNIC-IL-2 T/nT ratio and the mean value of IMT. However, the difference in T/nT ratio between atherosclerotic plaques with or without calcification on ultrasound was close to statistical significance (2.35 ± 0.68 vs 1.924 ± 0.55, p = 0.088) (Fig. 1.)
Fig. 1.
Mean values of T/nT ratio of 99mTc-HYNIC-IL-2 uptake in atherosclerotic plaques without and with calcifications (1.924 ± 0.55 vs 2.35 ± 0.68, p = 0.088)
The mean values of measured cytokines, acute phase proteins as well as calcium-phosphate parameters were significantly higher in the group of patients with ESRD as compared to the control group. The values of the lipid profile component, however, were not statistically significantly different between the ESRD patients and the control group (all data presented in Table 1).
Table 1.
The values of measured biochemical parameters in patients with ESRD and in the control group
| Parameter | Unit | ESRD patients | Control group | Reference range | p |
|---|---|---|---|---|---|
| Median (range) | Median (range) | ||||
| Haemoglobin | g/dl | 12.05 (8.4–16.1) | 14.30 (12.80–16.00) | 11–17 | <0.01 |
| White blood count | 103/μl | 5.93 (3.83–14.04) | 6.24 (4.15–7.71) | 4–10 | NS |
| Albumin | g/l | 41 (33–56) | 45 (45–50) | 35.0–50.0 | <0.01 |
| Creatinine | μmol/l | 745.2 (270.3–1134.6) | 60.15 (42.80–99.6) | 45.0–97.0 | <0.01 |
| Urea nitrogen | mmol/l | 18.50 (12.50–34.80) | 4.85 (3.4–6.9) | 1.7–8.3 | <0.01 |
| Phosphate | 1.69 (1.07–2.78) | 1.11 (0.77–1.41) | 0.87–1.45 | <0.01 | |
| Total calcium | 2.20 (1.80–2.56) | 2.36 (2.28–2.32) | 2.02–2.61 | 0.02 | |
| Fibrinogen | g/l | 6.36 (2.50–8.70) | 2.76 (2.1 3–3.81) | 1.8–3.5 | <0.01 |
| C-reactive protein (CRP) | mg/l | 3.87 (0.26–30.30) | 0.98 (0.21–5.1) | 0 – 3 | 0.02 |
| Interleukin-6 (IL-6) | pg/ml | 2.645 (0.67–19.81) | 0.52 (0.27–1.85) | 0.447–9.96 | <0.01 |
| Soluble IL-2 receptor (sIL-2R) | 2,987.5 (1,535–5,739) | 893 (699–1323) | 458–1997 | 0.01 | |
| Soluble intercellular adhesion molecule-1 (sICAM-1) | ng/ml | 304 (210–484) | 229.07 (194.35–283.63) | 115–306 | 0.01 |
| Amyloid A (SAA) | mg/l | 5.7 (1.7–145) | 2.3 (0.90–9.7) | 5.0–6.4 | 0.01 |
| Interleukin-18 (IL-18) | pg/ml | 522.7 (318.4–1437.2) | 286.35 (167.5–449.5) | 37–215 | <0.01 |
| Antibodies to human heat shock protein 60 (aHSP60) | ng/ml | 29.21 (8.54–267.32) | 23 (16.66–46.50) | No data | NS |
| Soluble CD40 ligand (sCD40L) | 9.39 (2.92–17.74) | 14.49 (7.62–22.11) | 1.13–3.13 | <0.01 | |
| Asymmetric dimethylarginine (ADMA) | μmol/l | 0.923 (0.267–1.476) | 0.579 (0.348–0.968) | 0.26–0.64 | 0.047 |
| Homocysteine | 24.8 (8.5–67.2) | 10.95 (5.3–17) | 3.6–15.0 | <0.01 | |
| Anti-oxidized LDL | 163.25 (65.26–1200) | 158.75 (37–1200) | No data | NS | |
| HDL cholesterol | mmol/l | 1.24 (0.86–2.35) | 1.30 (1.03–2.04) | 0.9–3.00 | NS |
| LDL cholesterol | 2.6 (1.49–5.42) | 3.17 (2.4–6.3) | 0.2–3.40 | NS | |
| Triglycerides | 1.805 (0.9–3.35) | 1.39 (0.94–4.83) | 0.2–2.30 | <0.01 | |
| Adiponectin | ng/ml | 16,846.5 (3,437–43,497) | 5,867 (4,080–13,346) | 865–21424 | <0.01 |
| Apolipoprotein A-I (apoA-I) | g/l | 1.55 (1.03–2.08) | 1.66 (1.37–2.05) | W 1.25–2.15 | NS |
| M 1.10–2.05 | |||||
| Apolipoprotein B (apoB) | 0.95 (0.56–1.58) | 0.96 (0.68–1.72) | W 0.55–1.25 | NS | |
| M 0.55–1.40 | |||||
| Apolipoprotein B/A-I (apoB/A-I) | – | 0.59 (0.32–1.27) | 0.61 (0.34–0.98) | W 0.3–0.9 | NS |
| M 0.55–1.0 | |||||
| Oxidized LDL | ng/ml | 49.52 (4.13–2340.64) | 41.41 (9.88–259.54) | 18–2261 | NS |
| Body mass index | kg/m2 | 25.40 (19.43–34.60) | 25.01 (20.82–36.57) | 18.5–24.99 | NS |
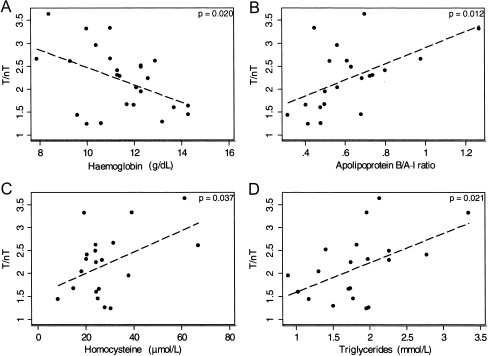
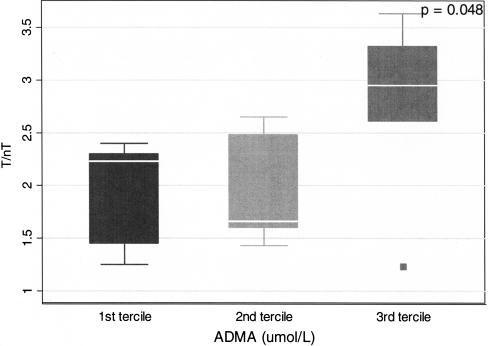
A statistically significant inverse relationship was found between scintigraphy results (T/nT ratio) and haemoglobin concentration (R = −0.21, p = 0.02) (Fig. 2a). Furthermore, the following parameters correlated significantly with T/nT ratio: apoB (R = 31, p = 0.008), apoB/apoA-I ratio (R = 0.29, p = 0.012) (Fig. 2b), homocysteine (R = 0.22, p = 0.037) (Fig. 2c) and triglycerides (R = 0.26, p = 0.021) (Fig. 2d). Moreover, ADMA concentration, which is considered one of the strongest risk factors for cardiovascular disease, was found to be significantly higher in the group of patients in the third tercile (patients with the highest values) of IL-2 uptake in scintigraphy (Fig. 3).
Fig. 2.
The correlation between serum concentrations of haemoglobin (a), apolipoprotein B/A-I ratio (b), homocysteine value (c), triglycerides (d) and T/nT ratio of 99mTc-HYNIC-IL-2 uptake within atherosclerotic plaque in ESRD patients
Fig. 3.
The differences of T/nT ratio of focal 99mTc-HYNIC-IL-2 uptake depending on ADMA value (terciles) in CCA walls in ESRD patients
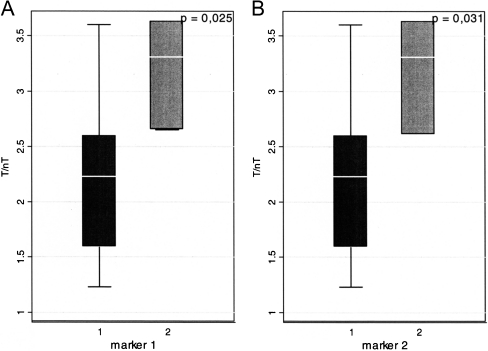
The multivariate analysis revealed that patients with a better profile of nutritional parameters (haemoglobin, albumin, adiponectin—all values above median) were characterized with lower T/nT ratio as compared with patients with a worse nutritional profile (haemoglobin, albumin, adiponectin—all values bellow median) (2.16 ± 0.68 vs 3.2 ± 0.5, p = 0.025) (Fig. 4a). Similarly, lower values of T/nT ratio were found in groups of patients characterized by all apoB, sCD40L and ADMA concentrations below median compared to concentrations above median (2.16 ± 0.68 vs 3.18 ± 0.52, p = 0.031) (Fig. 4b).
Fig. 4.
a Mean values of T/nT ratio of 99mTc-HYNIC-IL-2 uptake in patients with better (1) parameters of nutritional status (haemoglobin, albumin, adiponectin) in comparison with patients with worse (2) nutritional parameters (3.20 ± 0.5 vs 2.16 ± 0.68, p = 0.025). b Mean values of T/nT ratio of 99mTc-HYNIC-IL-2 uptake in patients with values of apoB, sCD40L and ADMA below (1) and above (2) median (2.16 ± 0.68 vs 3.18 ± 0.52, p = 0.031)
No correlations were found between scintigraphy results and cardiovascular events in anamnesis (2.40 ± 0.29 vs 2.23 ± 0.75, p = NS), classic cardiovascular risk factors, statin or other drug treatment. Only three patients declared active smoking; however, their mean T/nT was 2.60 ± 1.01 whereas in the non-smoking group it equalled 2.20 ± 0.67, p = NS.
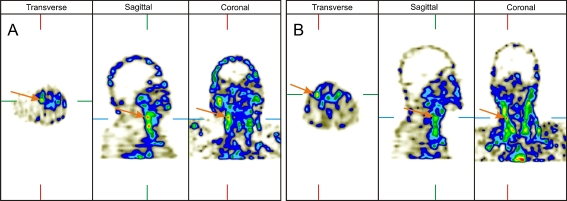
Examples of scintigraphy results obtained in a 54-year-old man on peritoneal dialysis with bilateral calcified carotid plaques and a 48-year-old man on peritoneal dialysis with bilateral non-calcified carotid plaques are presented in Fig. 5a, b.
Fig. 5.
The scintigraphy results in a 54-year-old man on peritoneal dialysis with bilateral calcified carotid plaques (a) and a 48-year-old man on peritoneal dialysis with bilateral non-calcified carotid plaques (b)
Discussion
Searching for methods of short- and long-term cardiovascular risk assessment is essential and involves many visualization techniques. Among them, various nuclear medicine techniques offer valuable and specific opportunities due to the wide range of available methods and tracers.
Currently in humans the easiest and most widely available nuclear medicine methods in assessing atherosclerotic plaques are visualizations of the peripheral arteries using SPECT or planar scintigraphy. Several attempts at atherosclerotic plaque imaging were unsuccessful since most of the tracers used did not confirm their clinical utility due to their toxicity [16], long time of tracer uptake [17], long clearance time [18], low values of achieved T/nT ratio [19, 20] or interactions with drugs used in the treatment of cardiovascular disease [21, 22]. However, some of them with the use of Annexin V as a marker of apoptosis, monocyte chemoattractant protein-1 (MCP-1) as a marker of macrophage infiltration [23], 111In-Z2D3 F(ab’)2 against proliferating smooth muscles [24] or IL-2 [6, 7] were promising. Among PET tracers the most advanced studies used 18F-fluorodeoxyglucose (FDG) and they confirmed increased uptake of that tracer in atherosclerotic plaques [25] while the arteries without atherosclerotic plaques did not take up 18F-FDG [26].
By now, two studies describing IL-2 use in CCA atherosclerotic visualization have been performed, but neither of them has clearly answered the question about the relationship between scintigraphic result and chronic inflammation, considered as a one of the most important cardiovascular risk factors.
Annovazzi et al. were the first investigators to employ 99mTc-IL-2 to visualize CCA plaques [6]. They demonstrated the correlation between three cytological parameters: the number of IL-2R-positive cells on histopathology, the absolute number of IL-2R-positive cells as well as the percentage of IL-2R-positive cells on flow cytometry and 99mTc-HYNIC-IL-2 uptake on SPECT. They did not reveal, however, any association between scintigraphy results and plaque echostructure or surface on ultrasound.
To the best of our knowledge, we were the first to publish a pilot study with CCA plaque visualization using 123I-IL-2 in ESRD patients with high cardiovascular risk [7]. The main findings in that study confirmed the potential usefulness of this tracer and applied examination techniques in visualization of the inflamed atherosclerotic lesions (100% sensitivity of scintigraphy in CCA plaque imaging). In that study we also found a correlation between the quantitative results of scintigraphy, the results of IMT measurement and the subsequent risk of cardiovascular event over the next 3 years of follow-up (data not published yet). These encouraging results inclined us towards testing this approach with a better (safer and cheaper) tracer in a larger group of ESRD patients with slightly lower cardiovascular risk, thus in patients who could take greater advantage of cardiovascular risk assessment. In the current study we failed to find a significant relationship between cardiovascular events, classic cardiovascular risk factors and IL-2 scintigraphy results. Indeed, the lack of correlation probably resulted from the small number of cardiovascular events during the observation time, lower patient inflammatory state in comparison with the 123I study and the relatively short time of observation (mean 14 months). The lack of a statistically important impact of classic cardiovascular risk factors on IL-2 scintigraphy in the current study in the first place resulted from a relatively small effect of the single factor in cardiovascular risk and the small groups for statistically important results in multifactor analysis.
In our present study, in addition to plaque localization and IMT measurements, we also assessed the presence of calcifications within the plaque. Surprisingly, we found that plaques with calcifications were characterized by a higher value of T/nT as compared to those without calcification (2.35 ± 0.68 vs 1.92 ± 0.55, p = 0.088). This observation may on the one hand be considered unusual, because many previous studies have shown that calcified plaques are “older”, usually with a lower degree of inflammation and thus more stable. On the other hand, it has been demonstrated repeatedly that plaque calcification is an active process with engagement of activated inflammatory cells and proinflammatory cytokines [27, 28].
The choice of the best biochemical test for cardiovascular risk assessment in everyday practice is difficult due to the overlapping of different diseases in the group of patients with high cardiovascular risk (and especially with chronic uraemia). However, some cytokines (e.g. IL-6, IL-18), acute phase proteins (e.g. CRP, fibrinogen) or components of the lipid profile (triglycerides, LDL-C, apoA-I, apoB) seem to be reasonable tools for assessment of the degree of inflammation and concomitant cardiovascular risk.
In the 123I-IL-2 study quoted above we failed to find any association between scintigraphy results and biochemical variables, probably because the number of participants was too small or due to different radiopharmaceutical properties of the isotopes.
In the present research, the analysis of the relationship between 99mTc-HYNIC-IL-2 scintigraphy and the results of the biochemical tests revealed the associations between T/nT and concentrations of haemoglobin, serum homocysteine, ADMA, triglycerides, apoB and apoB/apoA-I ratio. These correlations may indirectly confirm the link between systemic inflammation (and possibly inflammation activity within an atherosclerotic plaque) and scintigraphy results. Furthermore, in ESRD patients the high inflammatory state and signs of poor nutritional status correlate with higher cardiovascular risk [8]. The multifactor analyses of laboratory data show positive correlation between IL-2 uptake and low values for parameters of nutritional status (haemoglobin, albumin, adiponectin) as well as negative factors (ADMA, apoB, sCD40L). Those positive associations achieved in our study between atherosclerosis and inflammation indicate IL-2 scintigraphy as a potentially valuable method of assessing atherosclerotic plaque inflammatory state.
Conclusion
Scintigraphy with 99mTc-HYNIC-IL-2 can be considered a useful tool for visualizing the inflamed atherosclerotic (vulnerable) plaque within CCA in ESRD patients. The quantitative results of the carotid artery 99mTc-HYNIC-IL-2 scintigraphy correlated with serum concentration of certain biochemical markers of cardiovascular risk.
Performing scintigraphy with radiolabelled IL-2 in addition to currently available methods would seem to be a step toward a better assessment of cardiovascular risk by recognizing vulnerable patients through identification of the inflammatory process within an atherosclerotic plaque. This may enable earlier, more aggressive treatment, which may lead to the stabilization of potentially vulnerable plaques.
Acknowledgement
This work was supported by the Polish Committee for Scientific Research (KBN) within Research Project 2 P05B 003 28.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Malek TR. The main function of IL-2 is to promote the development of T regulatory cells. J Leukoc Biol. 2003;74:961–965. doi: 10.1189/jlb.0603272. [DOI] [PubMed] [Google Scholar]
- 2.Hoyer KK, Dooms H, Barron L, Abbas AK. Interleukin-2 in the development and control of inflammatory disease. Immunol Rev. 2008;226:19–28. doi: 10.1111/j.1600-065X.2008.00697.x. [DOI] [PubMed] [Google Scholar]
- 3.Annovazzi A, Biancone L, Caviglia R, Chianelli M, Capriotti G, Mather SJ, et al. 99mTc-interleukin-2 and (99m)Tc-HMPAO granulocyte scintigraphy in patients with inactive Crohn’s disease. Eur J Nucl Med Mol Imaging. 2003;30:374–382. doi: 10.1007/s00259-002-1069-x. [DOI] [PubMed] [Google Scholar]
- 4.Signore A, Chianelli M, Annovazzi A, Rossi M, Maiuri L, Greco M, et al. Imaging active lymphocytic infiltration in coeliac disease with iodine-123-interleukin-2 and the response to diet. Eur J Nucl Med. 2000;27:18–24. doi: 10.1007/PL00006657. [DOI] [PubMed] [Google Scholar]
- 5.Chianelli M, Mather SJ, Grossman A, Sobnak R, Fritzberg A, Britton KE, et al. (99m)Tc-interleukin-2 scintigraphy in normal subjects and in patients with autoimmune thyroid diseases: a feasibility study. Eur J Nucl Med Mol Imaging. 2008;35:2286–2293. doi: 10.1007/s00259-008-0837-7. [DOI] [PubMed] [Google Scholar]
- 6.Annovazzi A, Bonanno E, Arca M, D’Alessandria C, Marcoccia A, Spagnoli LG, et al. 99mTc-interleukin-2 scintigraphy for the in vivo imaging of vulnerable atherosclerotic plaques. Eur J Nucl Med Mol Imaging. 2006;33:117–126. doi: 10.1007/s00259-005-1899-4. [DOI] [PubMed] [Google Scholar]
- 7.Hubalewska-Dydejczyk A, Stompór T, Kalembkiewicz M, Krzanowski M, Mikolajczak R, Sowa-Staszczak A, et al. Identification of the inflamed atherosclerotic plaque using 123I-labeled interleukin-2 scintigraphy in high-risk peritoneal dialysis patients: a pilot study. Perit Dial Int. 2009;29:568–574. [PubMed] [Google Scholar]
- 8.Tonbul HZ, Demir M, Altintepe L, Güney I, Yeter E, Türk S, et al. Malnutrition-inflammation-atherosclerosis (MIA) syndrome components in hemodialysis and peritoneal dialysis patients. Ren Fail. 2006;28:287–294. doi: 10.1080/08860220600583625. [DOI] [PubMed] [Google Scholar]
- 9.Zoccali C, Benedetto FA, Mallamaci F, Tripepi G, Fermo I, Focà A, et al. Inflammation is associated with carotid atherosclerosis in dialysis patients. Creed Investigators. Cardiovascular Risk Extended Evaluation in Dialysis Patients. J Hypertens. 2000;18:1207–1213. doi: 10.1097/00004872-200018090-00006. [DOI] [PubMed] [Google Scholar]
- 10.Zoccali C, Benedetto FA, Maas R, Mallamaci F, Tripepi G, Malatino LS, et al. Asymmetric dimethylarginine, C-reactive protein, and carotid intima-media thickness in end-stage renal disease. J Am Soc Nephrol. 2002;13:490–496. doi: 10.1097/01.ASN.0000032548.18973.0F. [DOI] [PubMed] [Google Scholar]
- 11.Armstrong EJ, Morrow DA, Sabatine MS. Inflammatory biomarkers in acute coronary syndromes: part I: introduction and cytokines. Circulation. 2006;113(6):e72–e75. doi: 10.1161/CIRCULATIONAHA.105.595520. [DOI] [PubMed] [Google Scholar]
- 12.Armstrong EJ, Morrow DA, Sabatine MS. Inflammatory biomarkers in acute coronary syndromes: part II: acute-phase reactants and biomarkers of endothelial cell activation. Circulation. 2006;113(7):e152–e155. doi: 10.1161/CIRCULATIONAHA.105.595538. [DOI] [PubMed] [Google Scholar]
- 13.Armstrong EJ, Morrow DA, Sabatine MS. Inflammatory biomarkers in acute coronary syndromes: part III: biomarkers of oxidative stress and angiogenic growth factors. Circulation. 2006;113(8):e289–e292. doi: 10.1161/CIRCULATIONAHA.105.595546. [DOI] [PubMed] [Google Scholar]
- 14.Armstrong EJ, Morrow DA, Sabatine MS. Inflammatory biomarkers in acute coronary syndromes: part IV: matrix metalloproteinases and biomarkers of platelet activation. Circulation. 2006;113(9):e382–e385. doi: 10.1161/CIRCULATIONAHA.105.595553. [DOI] [PubMed] [Google Scholar]
- 15.Karczmarczyk U, Garnuszek P, Maurin M, Di Gialleonardo V, Galli F, Signore A, et al. Investigation of 99mTc-labelling of recombinant human interleukin-2 via hydrazinonicotinamide. Nucl Med Biol. 2010;37(7):795–803. doi: 10.1016/j.nucmedbio.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 16.Tsimikas S. Noninvasive imaging of oxidized low-density lipoprotein in atherosclerotic plaques with tagged oxidation-specific antibodies. Am J Cardiol. 2002;90(10C):22L–27L. doi: 10.1016/S0002-9149(02)02958-2. [DOI] [PubMed] [Google Scholar]
- 17.Mettinger KL, Larsson S, Ericson K, Casseborn S. Detection of atherosclerotic plaques in carotid arteries by the use of 123I-fibrinogen. Lancet. 1978;1(8058):242–244. doi: 10.1016/S0140-6736(78)90485-3. [DOI] [PubMed] [Google Scholar]
- 18.Lees AM, Lees RS, Schoen FJ, Isaacsohn JL, Fischman AJ, McKusick KA, et al. Imaging human atherosclerosis with 99mTc-labeled low density lipoproteins. Arteriosclerosis. 1988;8:461–470. doi: 10.1161/01.ATV.8.5.461. [DOI] [PubMed] [Google Scholar]
- 19.Iuliano L, Signore A, Vallabajosula S, Colavita AR, Camastra C, Ronga G, et al. Preparation and biodistribution of 99 m technetium labelled oxidized LDL in man. Atherosclerosis. 1996;126(1):131–141. doi: 10.1016/0021-9150(96)05888-1. [DOI] [PubMed] [Google Scholar]
- 20.Demacker PN, Dormans TP, Koenders EB, Corstens FH. Evaluation of indium-111-polyclonal immunoglobulin G to quantitate atherosclerosis in Watanabe heritable hyperlipidemic rabbits with scintigraphy: effect of age and treatment with antioxidants or ethinylestradiol. J Nucl Med. 1993;34:1316–1321. [PubMed] [Google Scholar]
- 21.Moriwaki H, Matsumoto M, Handa N, Isaka Y, Hashikawa K, Oku N, et al. Functional and anatomic evaluation of carotid atherothrombosis. A combined study of indium 111 platelet scintigraphy and B-mode ultrasonography. Arterioscler Thromb Vasc Biol. 1995;15:2234–2240. doi: 10.1161/01.ATV.15.12.2234. [DOI] [PubMed] [Google Scholar]
- 22.Minar E, Ehringer H, Dudczak R, Schöfl R, Jung M, Koppensteiner R, et al. Indium-111-labeled platelet scintigraphy in carotid atherosclerosis. Stroke. 1989;20:27–33. doi: 10.1161/01.STR.20.1.27. [DOI] [PubMed] [Google Scholar]
- 23.Ohtsuki K, Hayase M, Akashi K, Kopiwoda S, Strauss HW. Detection of monocyte chemoattractant protein-1 receptor expression in experimental atherosclerotic lesions: an autoradiographic study. Circulation. 2001;104(2):203–208. doi: 10.1161/01.cir.104.2.203. [DOI] [PubMed] [Google Scholar]
- 24.Carrió I, Pieri PL, Narula J, Prat L, Riva P, Pedrini L, et al. Noninvasive localization of human atherosclerotic lesions with indium 111-labeled monoclonal Z2D3 antibody specific for proliferating smooth muscle cells. J Nucl Cardiol. 1998;5(6):551–557. doi: 10.1016/S1071-3581(98)90108-8. [DOI] [PubMed] [Google Scholar]
- 25.Font MA, Fernandez A, Carvajal A, Gamez C, Badimon L, Slevin M, et al. Imaging of early inflammation in low-to-moderate carotid stenosis by 18-FDG-PET. Front Biosci. 2009;14:3352–3360. doi: 10.2741/3457. [DOI] [PubMed] [Google Scholar]
- 26.Rudd JH, Warburton EA, Fryer TD, Jones HA, Clark JC, Antoun N, et al. Imaging atherosclerotic plaque inflammation with [18F]-fluorodeoxyglucose positron emission tomography. Circulation. 2002;105(23):2708–2711. doi: 10.1161/01.CIR.0000020548.60110.76. [DOI] [PubMed] [Google Scholar]
- 27.Tintut Y, Patel J, Parhami F, Demer LL. Tumor necrosis factor-alpha promotes in vitro calcification of vascular cells via the cAMP pathway. Circulation. 2000;102:2636–2642. doi: 10.1161/01.cir.102.21.2636. [DOI] [PubMed] [Google Scholar]
- 28.Kaden JJ, Dempfle CE, Grobholz R, Fischer CS, Vocke DC, Kiliç R, et al. Inflammatory regulation of extracellular matrix remodeling in calcific aortic valve stenosis. Cardiovasc Pathol. 2005;14:80–87. doi: 10.1016/j.carpath.2005.01.002. [DOI] [PubMed] [Google Scholar]