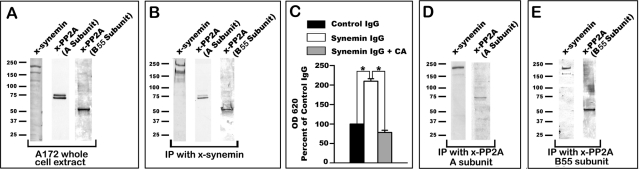
FIGURE 6:
Immunoprecipitation experiments with synemin and PP2A antibodies using A172 cell cytosolic proteins. (A) Western blots of A172 cells total protein extracts show the specificities of the antibodies used for immunoprecipitation; note that the synemin antibody recognizes two bands corresponding to α- and β-synemin. (B) Immunoprecipitation with synemin antibodies demonstrates that the A subunit and B55 subunit of PP2A are pulled down together with synemin. (C) Measurements of the phosphatase activity present in the proteins immunoprecipitated with control or synemin IgGs. In some experiments, 500 nM CA was added to the immunoprecipitated proteins. Immunoprecipitated proteins were incubated with a phosphopeptide substrate, and the Pi released was measured at OD 620 with a malachite green assay. Compared to control IgGs, synemin antibodies immunoprecipitated a phosphatase activity that can be inhibited with CA. Bars represent means ± SEM of three independent experiments; asterisks indicate significance at p ≤ 0.001. (D, E) Immunoprecipitation with antibodies against the A (D) and B55 (E) subunits of PP2A demonstrate that synemin pulls down together with these subunits.