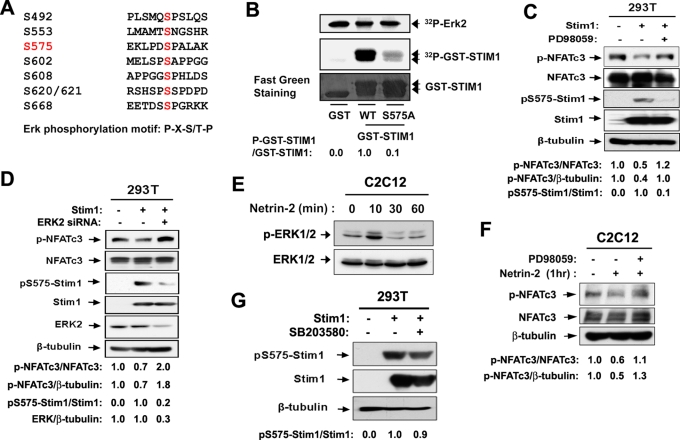
FIGURE 4:
Netrin-2 induces phosphorylation of Stim1 at serine 575 via ERK activation. (A) The consensus ERK phosphorylation motif and a potential phosphorylation site at serine 575. (B) In vitro phosphorylation of the putative ERK phosphorylation site of Stim1 by ERK. The wild-type GST-Stim1 encompassing amino acids 542–607 or the alanine mutant form of GST-Stim1 at serine 575 (S575A) was used as substrate, and phosphorylation was measured by 32P incorporation. GST serves as a negative control. The substrate levels were determined using fast green staining. p-GST-STIM1 and GST-STIM1 signals were quantified by densitometry. The signal ratio of GST-STIM1WT was set to 1. (C) 293T cells transfected with Stim1 were treated with PD98059 for 1 h and analyzed by immunoblotting. Signals of p-NFATc3, NFATc3, p575-Stim1, Stim1, and β-tubulin were quantified by densitometry, and the ratio from control transfectants was set to 1. (D) Lysates of 293T cells cotransfected with control or Stim1 expression vector plus a scrambled siRNA or ERK2 siRNA were subjected to immunoblotting with antibodies to p-NFATc3, NFATc3, pS575-Stim1, Stim1, ERK2, and β-tubulin as a loading control. Signals of p-NFATc3, NFATc3, ERK2, and β-tubulin were quantified by densitometry; ratios are reported under each lane at the bottom in arbitrary units, with control transfectants set to 1. (E) Lysates from C2C12 cells treated with netrin-2 for indicated times were immunoblotted with antibodies to p-ERK1/2 or ERK1/2. (F) C2C12 cells were incubated for 8 h in DM and treated with netrin-2 for 1 h. For the treatment with PD98059, cells were pretreated with PD98059 for 1 h, prior to the netrin treatment. Lysates were immunoblotted with indicated antibodies. Signals of p-NFATc3, NFATc3, and β-tubulin were quantified by densitometry, and the ratio from the control transfectant was set to 1. (G) 293T cells transfected with Stim1 were treated with SB203580 for 1 h and analyzed by immunoblotting with antibodies to pS575-Stim1, Stim1, and β-tubulin as a loading control. Signals of pS575-Stim1 and Stim1 were quantified by densitometry, and the ratio from the control transfectant was set to 1.