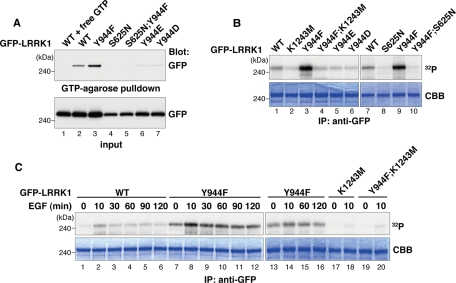
FIGURE 2:
Phosphorylation of LRRK1 at Tyr-944 reduces its kinase activity. (A) GTP-binding activity of LRRK1 mutants. Cos7 cells were transfected with WT GFP-LRRK1, GFP-LRRK1(Y944F), GFP-LRRK1(S625N), GFP-LRRK1(Y944F; S625N), GFP-LRRK1(Y944E), and GFP-LRRK1(Y944D), as indicated. Using GTP-agarose, GFP-LRRK1 protein was affinity purified from cytosolic extracts of transfected Cos7 cells in the presence or absence of 4 mM GTP (top). Protein input was confirmed by immunoblotting with anti-GFP antibodies (bottom). (B) Kinase activity of LRRK1 mutants. Cos7 cells were transfected with WT and LRRK1 mutants, as indicated. Immunoprecipitated samples were incubated with [γ-32P]ATP for 20 min at 30°C. Autophosphorylated LRRK1 was resolved by SDS–PAGE and stained with Coomassie blue to visualize total amounts of precipitated LRRK1 proteins. (C) The effect of EGF stimulation on LRRK1 kinase activity. Cos7 cells were transfected with WT GFP-LRRK1 and LRRK1 mutants, as indicated. After 16 h of serum starvation, cells were stimulated with EGF (100 ng/ml) for the indicated times. In vitro autophosphorylation assays were carried out as in B.