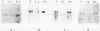Abstract
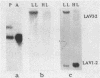
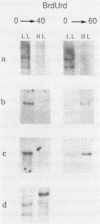
We have tested the hypothesis which stipulates that only early-replicating genes are capable of expression. Within one cell type of Physarum - the plasmodium - we defined the temporal order of replication of 10 genes which were known to be variably expressed in 4 different developmental stages of the Physarum life cycle. Southern analysis of density-labeled, bromodesoxyuridine-substituted DNA reveals that 4 genes presumably inactive within the plasmodium, were not restricted to any temporal compartment of S-phase: 1 is replicated in early S-phase, 2 in mid S-phase and 1 in late S-phase. On the other hand, 4 out of 6 active genes analysed are duplicated early, with the first 30% of the genome. Surprisingly, the two others active genes are replicated late in S-phase. By gene-dosage analysis, based on quantitation of hybridization signals from early and late replicating genes throughout S-phase, we could pinpoint the replication of one of these two genes at a stage where 80-85% of the genome has duplicated. Our results demonstrate that late replication during S-phase does not preclude gene activity.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernier F., Lemieux G., Pallotta D. Gene families encode the major encystment-specific proteins of Physarum polycephalum plasmodia. Gene. 1987;59(2-3):265–277. doi: 10.1016/0378-1119(87)90334-9. [DOI] [PubMed] [Google Scholar]
- Braun R., Mittermayer C., Rusch H. P. Sequential temporal replication of DNA in Physarum polycephalum. Proc Natl Acad Sci U S A. 1965 May;53(5):924–931. doi: 10.1073/pnas.53.5.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer B. J. When polymerases collide: replication and the transcriptional organization of the E. coli chromosome. Cell. 1988 Jun 3;53(5):679–686. doi: 10.1016/0092-8674(88)90086-4. [DOI] [PubMed] [Google Scholar]
- Brown E. H., Iqbal M. A., Stuart S., Hatton K. S., Valinsky J., Schildkraut C. L. Rate of replication of the murine immunoglobulin heavy-chain locus: evidence that the region is part of a single replicon. Mol Cell Biol. 1987 Jan;7(1):450–457. doi: 10.1128/mcb.7.1.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calza R. E., Eckhardt L. A., DelGiudice T., Schildkraut C. L. Changes in gene position are accompanied by a change in time of replication. Cell. 1984 Mar;36(3):689–696. doi: 10.1016/0092-8674(84)90349-0. [DOI] [PubMed] [Google Scholar]
- Christensen M. E., Moloo J., Swischuk J. L., Schelling M. E. Characterization of the nucleolar protein, B-36, using monoclonal antibodies. Exp Cell Res. 1986 Sep;166(1):77–93. doi: 10.1016/0014-4827(86)90509-4. [DOI] [PubMed] [Google Scholar]
- Dretzen G., Bellard M., Sassone-Corsi P., Chambon P. A reliable method for the recovery of DNA fragments from agarose and acrylamide gels. Anal Biochem. 1981 Apr;112(2):295–298. doi: 10.1016/0003-2697(81)90296-7. [DOI] [PubMed] [Google Scholar]
- Epner E., Rifkind R. A., Marks P. A. Replication of alpha and beta globin DNA sequences occurs during early S phase in murine erythroleukemia cells. Proc Natl Acad Sci U S A. 1981 May;78(5):3058–3062. doi: 10.1073/pnas.78.5.3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Furst A., Brown E. H., Braunstein J. D., Schildkraut C. L. alpha-Globulin sequences are located in a region of early-replicating DNA in murine erythroleukemia cells. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1023–1027. doi: 10.1073/pnas.78.2.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert D. M. Temporal order of replication of Xenopus laevis 5S ribosomal RNA genes in somatic cells. Proc Natl Acad Sci U S A. 1986 May;83(9):2924–2928. doi: 10.1073/pnas.83.9.2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman M. A., Holmquist G. P., Gray M. C., Caston L. A., Nag A. Replication timing of genes and middle repetitive sequences. Science. 1984 May 18;224(4650):686–692. doi: 10.1126/science.6719109. [DOI] [PubMed] [Google Scholar]
- Goldman M. A. The chromatin domain as a unit of gene regulation. Bioessays. 1988 Aug-Sep;9(2-3):50–55. doi: 10.1002/bies.950090204. [DOI] [PubMed] [Google Scholar]
- Gottesfeld J., Bloomer L. S. Assembly of transcriptionally active 5S RNA gene chromatin in vitro. Cell. 1982 Apr;28(4):781–791. doi: 10.1016/0092-8674(82)90057-5. [DOI] [PubMed] [Google Scholar]
- Guinta D. R., Tso J. Y., Narayanswami S., Hamkalo B. A., Korn L. J. Early replication and expression of oocyte-type 5S RNA genes in a Xenopus somatic cell line carrying a translocation. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5150–5154. doi: 10.1073/pnas.83.14.5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamelin M., Adam L., Lemieux G., Pallotta D. Expression of the three unlinked isocoding actin genes of Physarum polycephalum. DNA. 1988 Jun;7(5):317–328. doi: 10.1089/dna.1.1988.7.317. [DOI] [PubMed] [Google Scholar]
- Hand R. Eucaryotic DNA: organization of the genome for replication. Cell. 1978 Oct;15(2):317–325. doi: 10.1016/0092-8674(78)90001-6. [DOI] [PubMed] [Google Scholar]
- Holmquist G., Gray M., Porter T., Jordan J. Characterization of Giemsa dark- and light-band DNA. Cell. 1982 Nov;31(1):121–129. doi: 10.1016/0092-8674(82)90411-1. [DOI] [PubMed] [Google Scholar]
- Huberman J. A., Zhu J. G., Davis L. R., Newlon C. S. Close association of a DNA replication origin and an ARS element on chromosome III of the yeast, Saccharomyces cerevisiae. Nucleic Acids Res. 1988 Jul 25;16(14A):6373–6384. doi: 10.1093/nar/16.14.6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison N., Weintraub H. Localization of DNAase I-sensitive sequences to specific regions of interphase nuclei. Cell. 1985 Dec;43(2 Pt 1):471–482. doi: 10.1016/0092-8674(85)90177-1. [DOI] [PubMed] [Google Scholar]
- Jalouzot R., Toublan B., Wilhelm M. L., Wilhelm F. X. Replication timing of the H4 histone genes in Physarum polycephalum. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6475–6479. doi: 10.1073/pnas.82.19.6475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubbies M., Pierron G. Mitotic cell cycle control in Physarum. Unprecedented insights via flow-cytometry. Exp Cell Res. 1983 Nov;149(1):57–67. doi: 10.1016/0014-4827(83)90380-4. [DOI] [PubMed] [Google Scholar]
- Lyon M. F. X-chromosome inactivation and developmental patterns in mammals. Biol Rev Camb Philos Soc. 1972 Jan;47(1):1–35. doi: 10.1111/j.1469-185x.1972.tb00969.x. [DOI] [PubMed] [Google Scholar]
- Nader W. F., Isenberg G., Sauer H. W. Structure of Physarum actin gene locus ardA: a nonpalindromic sequence causes inviability of phage lambda and recA-independent deletions. Gene. 1986;48(1):133–144. doi: 10.1016/0378-1119(86)90359-8. [DOI] [PubMed] [Google Scholar]
- Pierron G., Durica D. S., Sauer H. W. Invariant temporal order of replication of the four actin gene loci during the naturally synchronous mitotic cycles of Physarum polycephalum. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6393–6397. doi: 10.1073/pnas.81.20.6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierron G., Sauer H. W., Toublan B., Jalouzot R. Physical relationship between replicons and transcription units in Physarum polycephalum. Eur J Cell Biol. 1982 Nov;29(1):104–113. [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Schedl T., Dove W. F. Mendelian analysis of the organization of actin sequences in Physarum polycephalum. J Mol Biol. 1982 Sep;160(1):41–57. doi: 10.1016/0022-2836(82)90130-9. [DOI] [PubMed] [Google Scholar]
- Smithies O. The control of globin and other eukaryotic genes. J Cell Physiol Suppl. 1982;1:137–143. doi: 10.1002/jcp.1041130421. [DOI] [PubMed] [Google Scholar]
- TAYLOR J. H. Asynchronous duplication of chromosomes in cultured cells of Chinese hamster. J Biophys Biochem Cytol. 1960 Jun;7:455–464. doi: 10.1083/jcb.7.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. H. Origins of replication and gene regulation. Mol Cell Biochem. 1984;61(2):99–109. doi: 10.1007/BF00222489. [DOI] [PubMed] [Google Scholar]
- Trempe J. P., Lindstrom Y. I., Leffak M. Opposite replication polarities of transcribed and nontranscribed histone H5 genes. Mol Cell Biol. 1988 Apr;8(4):1657–1663. doi: 10.1128/mcb.8.4.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]