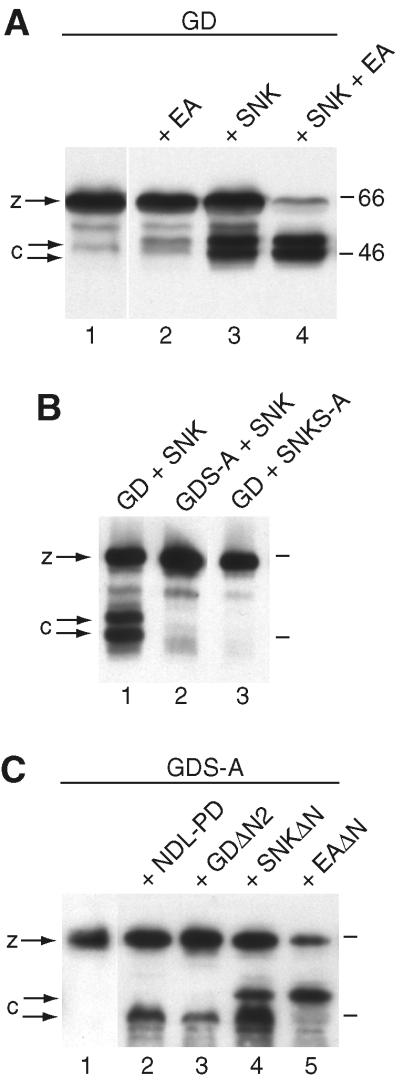
Figure 3.
Cleavage of GD in vitro. Western blots with anti-GD antibody to detect HA-tagged GD in culture medium of transfected S2 cells. (A) GD zymogen (z) is efficiently cleaved to generate two C-terminal forms (c) at 46 kDa and 50 kDa, when expressed with the Snake zymogen (lane 3) or both Snake and Easter zymogens (lane 4) but not when expressed with just the Easter zymogen (lane 2). These cleaved forms are also recognized by an anti-HA antibody (not shown). (B) Cleavage of GD (lane 1) requires the catalytic activity of GD itself (lane 2) and Snake (lane 3). (C) GDS-A is cleaved primarily to the 46-kDa form when expressed with Nudel's protease domain NDL-PD (lane 2) or GDΔN2 (lane 3); to both the 46- and 50-kDa forms when expressed with SNKΔN (lane 4); or to primarily the 50-kDa form when expressed with EAΔN (lane 5).