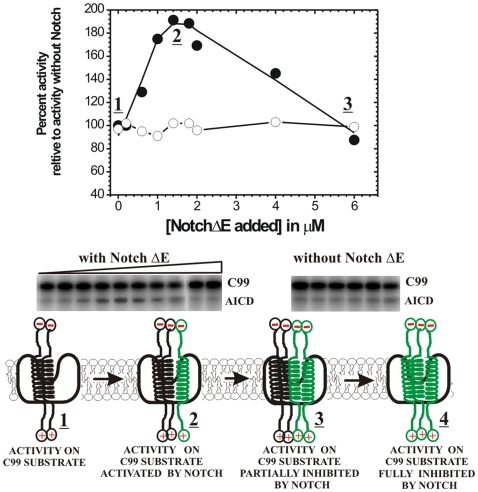
Figure 6. NotchΔE substrate can activate γ-secretase activity on C99 substrate.
γ-Secretase activity in CHAPSO enriched membranes was measured using half-saturating C99 substrate ([C99] = 0.45 µM, fresh after purification) in the presence of increasing concentration of NotchΔE substrate (•), and in identical control assays without NotchΔE substrate (O). The AICD production was measured using 125-I labeled C99 substrate and autoradiography as shown on the gel strips (125-I assay was used instead of western blot since both substrates were purified using antiflag M2 epitopes, see methods). Different interactions between γ-secretase and its C99 (black helix) or NotchΔE (green helix) substrates can be illustrated using a model mechanism. C99 substrate can be shown as a transmembrane helix [42], while γ-secretase can be shown as a bowl-shaped membrane-imbedded complex [19]. The underlined numbers connect the different complexes with the corresponding activity range on the graph. In a simple scenario, of one enzyme binding one substrate, NotchΔE and C99 substrates could be only competitive inhibitors [62]. We find that NotchΔE substrate can activate γ-secretase reaction on C99 substrate (1). Such scenario can happen only if γ-secretase can bind both substrates at the same time (2). NotchΔE substrate shows competition with C99 substrate only when its concentration is several folds higher than C99 concentration (3). Extrapolation of the presented profile shows that close to 10 µM of NotchΔE substrate would be needed for a full inhibition (4).