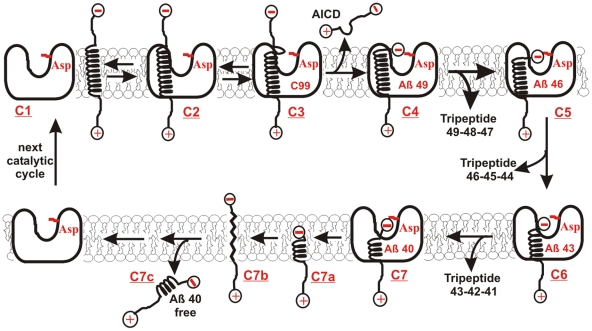
Figure 10.
Steps in the catalytic cycle of γ-secretase. The model illustrates the basic biophysical principles of processive cleavages and intramembrane proteolysis [11], [37], [40], [48], [49], [51]. C99 substrate can be shown as a transmembrane helix [42], while γ-secretase can be shown as a bowl-shaped membrane-imbedded complex with its active site aspartates in the central aqueous cavity [11], [18], [19]. The initial AICD cleavage (Fig. 1) takes place between amino acids 48–49 or 49–50 [37], just under the membrane surface [42], in a dynamic section that has a tendency to destabilize the transmembrane helix ((C1->C4), [58]). The result is a soluble AICD fragment, and a hydrophobic Aβ fragment with its negatively charged carboxyl-terminal trapped below the membrane surface (C3->C4). Thus, the negatively charged carboxyl-terminal is in an energy gap that is forcing it to the interface between the hydrophobic enzyme core and the hydrophilic central aqueous cavity. The opposing force comes from the hydrogen bonds that tend to stabilize the transmembrane helix (C4). The Aβ peptides have a highly dynamic structure that can vary from α-helix to random-coil [51]–[55], [57]. Such structural changes can drag small parts of the hydrophobic Aβ peptides to the active site aspartates following the negatively charged carboxyl-terminus in the central aqueous cavity ((C4->C7), [11]). Thus, the whole process can be driven by entropy and/or by repulsive forces between negative charges on the active site aspartates and the carboxyl-terminal on the nascent Aβ [51]–[54], [57]. There is no need for active use of cell's energy. The result is a sequence of processive cleavages of hydrophobic tri-peptides [48] that does not require a full exposure of the hydrophobic substrate to the aqueous catalytic site [11]. The initial cleavage at 49–50 site leads to Aβ 49–46–43–40 sequence, while the initial cleavage at 48–49 site leads to Aβ 48–45–42–38 sequence [37], [40], [48], [49]. It is very important to realize that the most frequent end-products Aβ 1–40 and Aβ 1–42 have more than a half of the original hydrophobic transmembrane helix of C99 (C6->C7). Such products are highly unlikely to spontaneously dissociate from the hydrophobic γ-secretase to the hydrophilic extracellular space (C7c). Furthermore, the peptides are too short to form a transmembrane helix (C7a) [62], while the fully extended structures (C7b) can not be stabile due to unsatisfied hydrogen bonds in the peptide backbone [62]. For the same reasons the nascent Aβ-peptides (C1->C6) can not be spontaneously released from γ-secretase. The hydrophobic Aβ products can dissociate from γ-secretase only by interacting with a carrier protein, or by forming an Aβ bundle as in Fig. 11. The carrier protein is expected to facilitate catalytic rates since dissociation of Aβ products is the rate-limiting step (Fig. 1, and Fig. S1). Thus, possible candidates for the carrier protein can be the proteins identified by He and coauthors [93], apo-lipoprotein E [5], PrP C [94], or some other surface proteins [60].