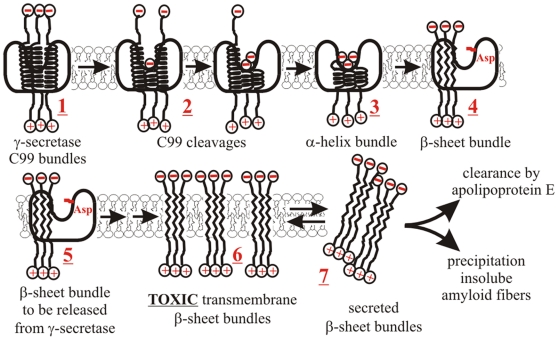
Figure 11. Multiple C99 molecules bound to γ-secretase can facilitate the pathogenesis.
Multiple C99 molecules bound to γ-secretase can affect the catalytic mechanism and contribute to the neurotoxic events. Multiple C99 molecules bound to the enzyme (1) could interact just as free C99 molecules [42]–[46]. Such interactions can influence the initial AICD cleavage and thus control the difference between Aβ 49–46–43–40 or Aβ 48–45–42 cleavage paths (Fig. 10). If multiple C99 molecules are cleaved in parallel, the result will be a bundle of nascent Aβ peptides (3), or even a mixed bundle of C99 and nascent Aβ peptides (2). All of those interactions can be affected by the same structural forces that control interactions between Aβ peptides in free solution. Thus, there could be a preferred number of peptides in the bundle [52], [53], and a preferred ratio between Aβ40, Aβ42, and the longer Aβ peptides [56]. Any of those can affect dynamic structural changes that control the processive cleavages, and ultimately the type of Aβ products (Fig. 10). Packed together the nascent Aβ peptides can undergo a series of structural changes so that their β-genic amino acids (Thr, Val, Ile) can initiate formation of extended β-sheet bundles (3->4) [51]–[54], [57], [58]. This can drive transition from the α-helix structure of C99 to the β-sheet structure of Aβ oligomers [51]–[54], [57], [58]. The whole process can be chaperoned and accelerated by the enclosure within the enzyme structure. Some functional and evolutional links have been observed between chaperones and rhomboid intramembrane proteases [95], [96]. Unlike single amyloid peptides (Fig. 10), the hydrophobic β-sheet bundles can be easily released into the lipid bilayer (5–>6). The bundles can be stabilized by hydrogen bonding between the peptides' backbones so that their hydrophobic amino acids can face the lipid bilayer [51]. The released β-sheet bundles can accumulate to toxic levels by causing disruption of membrane integrity (i.e. fluidity, lipid rafts and ion gradients [51], [97]). Thus, the neurotoxic processes can start directly in the membrane where toxic amyloid peptides are produced, rather than in the extracellular space as it was suggested in the original amyloid hypothesis and its subsequent derivatives [51]. Extracellular amyloid fibrils can be the end result of chronic toxic overload and the final membrane breakdown (7) [51].