Abstract
Lactation ketosis is a recognised disorder in postpartum lactating cows where a negative energy balance develops because the energy demands of milk production exceed the energy capacity of the animal. Rarely, nursing women can develop problems with lactation ketosis when their glycogen stores are depleted, causing the body to turn to gluconeogenesis as an energy substrate for galactopoiesis. The authors describe the case of a breastfeeding woman admitted to hospital and made nil per os (NPO) to treat a bowel obstruction. She did not receive intravenous glucose and 3 days postadmission developed a dangerous starvation ketosis (venous pH of 6.64). She was treated with intravenous dextrose, bicarbonate as well as cessation of breastfeeding and recovered quickly. Only four previous reports describe human lactation ketosis and this is the first iatrogenic case reported to our knowledge. It highlights the importance of addressing the unique caloric requirements of nursing women, especially when they are kept NPO.
Background
To our knowledge, this is the first case of iatrogenically caused lactation ketoacidosis in the medical literature. There have been four other case reports of human lactation ketoacidosis, but each of these was associated with different physiological stressors. We feel that this case report is relevant as it identifies important nutritional and physiological differences in the care of postpartum lactating women that can sometimes be overlooked as we infrequently encounter this patient population on non-obstetric hospital wards.
Case presentation
A 35-year-old postpartum woman presented to the emergency department with an 18 h history of nausea, vomiting and intermittent generalised abdominal pain. Her medical history included endometriosis, Crohn’s disease, caesarian section and multiple previous small bowel obstructions. She had no history of diabetes. The patient was diagnosed with a recurrent small bowel obstruction and was admitted to hospital under the general surgery service. Of note, she had been nursing her 3-week-old infant when she presented to medical attention and continued to do so while in hospital.
The bowel obstruction (believed to be due to adhesions) was managed conservatively by keeping the patient NPO (nil per os) and administering maintenance intravenous normal saline with potassium replacement.
On the third day of admission, the patient’s symptoms had improved and the decision was made to advance her diet to clear fluids. The same morning, bloodwork showed a calculated HCO3− of 7 and a venous pH of 6.64. A subsequent arterial blood gas revealed a pH of 7.15, a PCO2 of 13 and HCO3− of 4.
The patient was reassessed at this time. She reported two episodes of diarrhoea since the previous evening and stated that she felt well other than symptoms of mild shortness of breath. She had continued to breastfeed her infant since coming to hospital and had just started the newly ordered clear fluid diet after not having eaten for a total of 4 days. She denied chest pain, light-headedness, abdominal pain, nausea, vomiting and the use of alcohol or new medications. Her blood pressure was 122/72, temperature was 36.7°C, heart rate was 95, respiratory rate was 24 and she had an O2 saturation of 97% on room air. Head and neck, cardiovascular and abdominal examinations were normal. Her respiratory examination revealed tachypnea and five to six word dyspnoea.
Investigations
The patient’s repeat bloodwork at this time is summarised in table 1. Analysis of these values revealed that the difference between the change in the anion gap from normal corrected for albumin (20–9) and the change in bicarbonate concentration from normal (24–4) (the so-called δ gap) was 9, indicating a mixed anion gap metabolic acidosis (AGMA) and a normal anion gap metabolic acidosis (NAGMA). Calculations also revealed appropriate respiratory compensation for the observed metabolic acidosis (expected PaCO2 by Winter’s formula=14 +/−2; measured PaCO2=13). We concluded that the patient was in a state of starvation ketoacidosis (β-hydroxybutarate level 9.27 mmol/l) exacerbated by the metabolic demands of lactation and possible residual insulin resistance of pregnancy (AGMA). In addition, a component of bicarbonate loss due to diarrhea as well as third spacing of bicarbonate resulting from decreased intestinal motility was thought to be present (NAGMA).
Table 1.
Patient’s laboratory values on the morning of the third day of admission.
| Na+ | 135 mmol/l |
| K+ | 4.5 mmol/l |
| Cl− | 111 mmol/l |
| Creatinine | 84 umol/l |
| Albumin | 32 g/l |
| Blood glucose | 6.3 mmol/l |
| Lactate | <1.0 mmol/l |
| Salicylate | <1.0 mmol/l |
| β-hydroxybutarate | 9.27 mmol/l |
| Arterial pH | 7.15 |
| Arterial PCO2 | 13 |
| Arterial HCO3− | 4 |
| Anion gap | 20 |
Differential diagnosis
The differential diagnosis of an AGMA includes:
-
▶
lactic acidosis
-
▶
ketoacidosis (diabetic, alcoholic, starvation)
-
▶
renal failure
-
▶
ingestions (salicylate, ethylene glycol, methanol, paraldehyde, metformin, isoniazid)
The differential diagnosis of a NAGMA includes:
-
▶
diarrhoea
-
▶
renal tubular acidosis
-
▶
pancreatic fistula, enteric diversion of urine
-
▶
hyperalimentation
-
▶
volume expansion with normal saline
-
▶
acetazolamide use
Treatment
As a result of the profound acidaemia, the patient was transferred to the step-down unit on the same day (postadmission day 3). She stopped breastfeeding at this time and was treated with 150 mmol of sodium bicarbonate in 500 ml of D5W. Her intravenous fluids were subsequently switched from normal saline at 150 ml/h to 2/3 1/3 dextrose normal saline at 150 ml/h. Another dose of 50 mmol of sodium bicarbonate was later given.
Outcome and follow-up
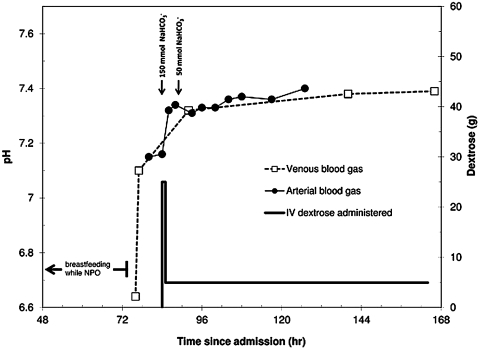
Over the next 24 h, the patient’s tachypnea improved and her blood gases started to normalise. After 48 h of treatment, her arterial blood gas showed a pH of 7.40, PaCO2 of 33 and HCO3− of 20. She remained in hospital for 2 additional days, tolerated a regular diet and was subsequently discharged home (see figure 1 for a complete timeline).
Figure 1.
Timeline of patient’s course in hospital. The patient’s pH improved quickly with the cessation of breastfeeding and continued to normalise with the administration of intravenous bicarbonate and dextrose.
Discussion
Ketoacidosis is a type of anion gap metabolic acidosis. It is seen in diabetic ketoacidosis, alcoholic ketoacidosis and starvation ketoacidosis.1
The metabolic acidosis seen in starvation ketoacidosis is a result of decreased insulin secretion leading to the mobilisation of free fatty acids, causing their incomplete oxidation and the accumulation of acetoacetate and β-hydroxybutyrate. The acidosis develops when glycogen stores are depleted and it is usually mild.2 More severe cases of starvation ketosis have been reported when a concomitant physiologic stressor exists,3 but it is uncommon for starvation ketosis to result in significant acidosis because of the presence of insulin.4 In pregnancy, however, there can be a more severe response to ketosis due to the insulin resistance caused by placental hormones.
Lactation ketosis is a recognised disorder in lactating cows that appears postpartum and is well described in the veterinary medicine literature. In this condition, there is a negative energy balance where the energy requirements of milk production exceed the energy capacity of the animal. This condition is called bovine ketosis and is common in ruminants because they rely almost exclusively on hepatic gluconeogenesis as a glucose source for milk production.5 In the postpartum period, the rate of hepatic gluconeogenesis in these animals doubles to meet the demands of lactation; however, there is no increase in dietary glucose supply. This energy imbalance is exacerbated in high-yield dairy cows.5
Lactating women, too, rely almost exclusively on gluconeogenesis as an energy substrate for milk production when they have been fasting for >42 h since hepatic glycogen stores are essentially depleted at this time. As a result, fasting lactating women are at risk to develop a more profound metabolic acidosis than their fasting non-lactating counterparts when their caloric needs are not met.6
We believe that our patient’s presentation parallels the case of lactation ketosis in cows. Specifically, the metabolic demands of lactation likely overwhelmed our patient’s glucose stores because she had been kept NPO without intravenous glucose replacement for more than 2 days. As previously described, the body is normally well equipped to deal with short-term fasting.2 In this particular scenario, however, the fact that our patient continued to breastfeed became the stressor that tipped the energy balance. Moreover, these metabolic derangements could have been exacerbated by residual insulin resistance of pregnancy.
We chose to treat our patient’s severe metabolic acidosis with sodium bicarbonate. Significant clinical debate exists as to whether or not this therapy should ever be given to patients with anion-gap metabolic acidosis. In fact, bicarbonate administration is not generally recommended in these patients because it may result in worsened intracellular and cerebral acidosis, and because there may be protective effects on some organs conferred by the acidosis.7 On the other hand, profound acidosis is associated with decreased myocardial contractility, and patients who suffer cardiac arrest with this level of acidosis are extremely difficult to resuscitate. There is no research on patients with a pH in the range of our patient’s. We opted to treat this patient with bicarbonate given the severity of her acidosis and her tachypnoea, but the wisdom of that choice is open to interpretation.
To our knowledge, there have been four cases of human lactation ketoacidosis reported in the medical literature.8–11 In all these cases, a physiological stressor existed that pushed each lactating patient into an unexpectedly serious acidosis (three women were dieting while breastfeeding and the other was breastfeeding twins).
Our report differs from each of these cases for a number of reasons. To begin, all other patients described presented to emergency departments with dyspnoea, whereas our patient’s acidosis was discovered on routine bloodwork in hospital. Furthermore, this case is unique as it describes iatrogenic lactation ketoacidosis. It was caused by the decision to treat a lactating patient’s bowel obstruction with complete bowel rest and a failure to provide intravenous glucose supplementation during this time.
Previous case reports of lactation ketoacidosis have made recommendations about the importance of avoiding dieting while breastfeeding. To our knowledge, no recommendations have been made about the specific care that lactating women require when NPO diets are ordered in hospital.
This case illustrates the importance of ensuring that intravenous glucose is provided to all patients requiring bowel rest. Furthermore, it highlights the need to consider the special nutritional demands of lactating women as this patient population has the potential to rapidly develop profound starvation ketosis – a phenomenon we rarely think about in the average patient that is kept NPO.
Although lactation ketoacidosis is an uncommon diagnosis to make, it should be considered and treated early to prevent potentially significant morbidity and mortality in an otherwise young and healthy patient population.
Learning points.
-
▶
Lactation ketoacidosis is a potentially dangerous form of starvation ketosis seen in postpartum lactating women where the metabolic demands of breastfeeding cause a profound metabolic acidosis in the context of nutritional deficits and residual insulin resistance of pregnancy.
-
▶
Fasting lactating women are at a greater risk to develop a profound metabolic acidosis than their fasting non-lactating counterparts when their caloric needs are not met.
-
▶
Lactating women have increased caloric demands that should be addressed when the decision is made to keep them NPO for medical reasons.
Acknowledgments
The authors would like to thank Dr Marco Sivilotti for editorial advice.
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.Shannon M. Acid-base, fluid, and electrolyte balance. In: Shannon M, Borron SW, Burns M, eds. Haddad and Winchester’s Clinical Management of Poisoning and Drug Overdose. Fourth Edition Philadelphia, PA: Saunders Elsevier; 2007 [Google Scholar]
- 2.Owen OE, Caprio S, Reichard GA, Jr, et al. Ketosis of starvation: a revisit and new perspectives. Clin Endocrinol Metab 1983;12:359–79 [DOI] [PubMed] [Google Scholar]
- 3.Toth HL, Greenbaum LA. Severe acidosis caused by starvation and stress. Am J Kidney Dis 2003;42:E16–9 [DOI] [PubMed] [Google Scholar]
- 4.Frise C, Mackillop L. Starvation ketosis in pregnancy. Proceedings of the Society of Endocrinology BES 2010, 15–18 March 2010, Manchester, UK: Endocrine; Abstracts 21:P143 [Google Scholar]
- 5.Bell AW, Bauman DE. Adaptations of glucose metabolism during pregnancy and lactation. J Mammary Gland Biol Neoplasia 1997;2:265–78 [DOI] [PubMed] [Google Scholar]
- 6.Mohammad MA, Sunehag AL, Chacko SK, et al. Mechanisms to conserve glucose in lactating women during a 42-h fast. Am J Physiol Endocrinol Metab 2009;297:E879–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gehlbach BK, Schmidt GA. Bench-to-bedside review: treating acid-base abnormalities in the intensive care unit - the role of buffers. Crit Care 2004;8:259–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chernow B, Finton C, Rainey TG, et al. “Bovine ketosis” in a nondiabetic postpartum woman. Diabetes Care 1982;5:47–9 [DOI] [PubMed] [Google Scholar]
- 9.Altus P, Hickman JW. Severe spontaneous ‘bovine’ ketoacidosis in a lactating woman. J Indiana State Med Assoc 1983;76:392–3 [PubMed] [Google Scholar]
- 10.Heffner AC, Johnson DP. A case of lactation “bovine” ketoacidosis. J Emerg Med 2008;35:385–7 [DOI] [PubMed] [Google Scholar]
- 11.Sandhu HS, Michelis MF, DeVita MV. A case of bovine ketoacidosis in a lactating woman. NDT Plus 2009;2:278–9 [DOI] [PMC free article] [PubMed] [Google Scholar]