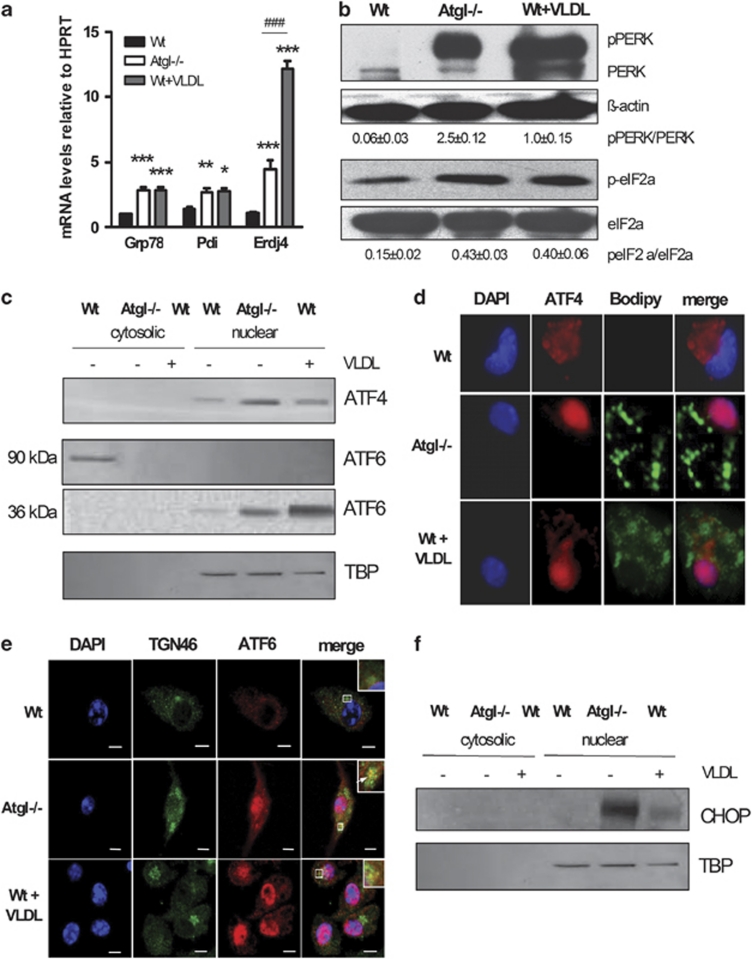
Figure 1.
TG accumulation triggers ER stress via activation of the PERK and ATF6 pathways. (a) Total RNA was isolated from Wt, Atgl–/– and VLDL-loaded Wt macrophages. GRP78/BiP, Pdi and ERdj4 mRNA levels, including normalization to hypoxanthine-guanine phosphoribosyltransferase (HPRT), were determined by real-time PCR. Data are expressed as mean values (n=4–6) of two independent experiments±S.E.M. *P<0.05, **P≤0.01, ***P≤0.001; ###P≤0.001. (b) Cytosolic fractions (40 μg protein per lane) of Wt, Atgl–/– and VLDL-loaded Wt macrophages were resolved by SDS-PAGE and protein expression was analyzed using specific antibodies against PERK and eIF2α. The expression of β-actin was used as loading control. Data are expressed as the ratios of pPERK/PERK and p-eIF2α/eIF2α from three independent experiments±S.E.M. (c) Cytosolic (40 μg protein per lane) and nuclear fractions (35 μg protein per lane) of Wt, Atgl–/– and VLDL-loaded Wt macrophages were resolved by SDS-PAGE and protein expression of ATF4 and ATF6 was determined by western blotting. TATA-binding protein (TBP) was used as nuclear fraction marker. (d) ATF4 and (e) ATF6 processing was analyzed after fixing and incubating the macrophages with specific antibodies. (d) Lipid droplets were stained with BODIPY 493/503. (e) Anti-TGN46 antibody was used as Golgi marker. (d and e) Cells were incubated with anti-rabbit Alexa-Fluor594 antibody and mounted in Vectashield/DAPI to visualize the nucleus. Representative images taken by fluorescence microscopy are shown. (f) CHOP protein expression was determined in nuclear fractions of Wt, Atgl–/– and VLDL-loaded Wt macrophages. TBP was used as nuclear fraction marker