Abstract
Racoleus trichophorus gen. sp. nov. is described for a tropical sterile filamentous lichenized fungus which overgrows various crustose lichens on bark. It shares some features with Cystocoleus and Racodium, but is unique in having non-lichenized long lateral spines. The genus, which is known from China, the Ivory Coast, and Peru, is of uncertain systematic position; on the basis of morphological similarities, however, it may be referred to “? Capnodiales (incertae sedis)” ad interim. In addition, the nomenclature and typification of the monotypic genera Cystocoleus and Racodium are reviewed, and lectotypes selected for the type of each. The available information on the ecology and distribution of these two genera is also summarized, and scanning electron micrographs (SEM) of all three species are presented for the first time.
Keywords: Ascomycota, Capnodiales, Coenogonium, lichens, Racoleus trichophorus, scanning electron microscopy, tropical fungi
INTRODUCTION
The enigmatic sterile filamentous lichens placed in Cystocoleus and Racodium are characterized by fungal hyphae, which surround a filament of the green alga Trentepohlia. The algal filaments determine the shape, and the enveloping hyphal layer is generally only single, the hyphae being parallel to the axis with elongate rectangular cells in Racodium, and irregularly twisted around the algal filament in Cystocoleus. Both genera comprise a single species, and occur, often together, on inclined to vertical siliceous rocks in recesses where it is cool and there is no direct rain but a high humidity. They are scarcely distinguishable macroscopically. Nevertheless, despite their anatomical similarity, molecular data have now shown that the two genera are not part of a single monophyletic group (Muggia et al. 2008). Both were found to belong to Capnodiales, with Racodium rupestre basal to the clade containing Cystocoleus ebeneus, which is close to Mycosphaerellaceae. These results have been confirmed by subsequent molecular phylogenetic analyses (Crous et al. 2009, Ruibal et al. 2009) with Cystocoleus now being recognised as a member of Teratosphaeriaceae and the more basal Racodium as incertae sedis.
A similar filamentous method of forming a lichen structure is seen otherwise only in a few genera. In Coenogonium, a leaf and bark dwelling member of the Gyalectaceae, the photobiont is a species of Trentepohlia in most species but can also be a filamentous species of Physolinum; in the corticolous sterile genus Pyrenothrix (syn. Lichenothrix) the filamentous photosynthetic partner belongs to the cyanobacterial genus Scytonema; Ephebe, a genus mainly of riverside rocks and belonging to the Ephebaceae, where the photosynthetic partner is Stigonema; and also in some species of the mainly tropical basidiomycete genus Dictyonema where the photosynthetic partner also belongs to Scytonema. A Physolinum forming lichen-like threads in which the algal cells can be in two or more rows, has also been documented from a dimly lit limestone cave, but the fungus involved has not been identified (Davis et al. 1989). In general, filamentous lichen associations are extremely rare, and no new genus of filamentous lichens has been described since the 19th century.
Here we describe a third genus of sterile filamentous lichens, Racoleus, for a tropical bark-inhabiting species known to R.S. for over 50 years, and differing in the development of long hair-like lateral spines which are not seen in Cystocoleus and Racodium, and also in the arrangement of the hyphae and the way they interlock. In addition, we take the opportunity to present some other observations aimed at clarifying and fixing the nomenclature and typification of Cystocoleus ebeneus and Racodium rupestre.
MATERIALS AND METHODS
Microscopic examinations were made with either a Wild or an Olmpus BH2 research microscope, both fitted with drawing tubes and the latter with a Nikon Coolpix 4500 digital camera and Nomarski interference contrast optics. All measurements were made in water mounts.
Scanning electron micrographs were prepared from air-dried specimens which had been gold-coated during rotation under vacuum, and examined in a Stereoscan (Cambridge Scientific Instruments) operating at 30 kv.
DNA extraction was attempted on a fragment of the Ivory Coast isotype of Racoleus trichophorus in GZU, carefully removed under a dissecting microscope, using the method of Cubero et al. (1999), but was unsuccessful. Molecular methods and results obtained with Cystocoleus ebeneus and Racodium rupestre have been reported separately (Muggia et al. 2008).
Specimen citations for Cystocoleus ebeneus and Racodium rupestre are restricted to those discussed in relation to typifications or distributed in exsiccatae.
TAXONOMY
Racoleus R. Sant. & D. Hawksw., gen. nov.
MycoBank MB561239
Etymology: From the generic names Rac[-odium] and [Cysto]-coleus, with which the genus has some features in common.
Similis Racodiis rupestris, sed differt in cellulis verrucosis et in spinulis lateralis non-lichenibus instructis.
Typus: Racoleus trichophorus R. Sant. & D. Hawksw. 2011.
Thallus superficial, fluffy, brown, filamentous. Photobiont Trentepohlia, single filaments of which are ensheathed by fungal hyphae. Filaments suberect to decumbent or spreading, sympodially branched, outer wall undulating and irregularly corrugated, with numerous lateral spines. Hyphae in a single layer surrounding the algal filament, orientated vertically along and always parallel to the axis of the filament, brown, septate, thick-walled, uneven and undulate to corrugated, not ornamented. Spines arising at broadly acute to almost right angles to the vertical axis, brown, stiff, thick-walled, smooth-walled, not ornamented or corrugated. Conidiogenous cells and conidia unknown.
Observations: While in the absence of any sexual state and molecular data no definite opinion on the systematic position of the new genus can be expressed, in view of the similarities to both Cystocoleus and Racodium it seems likely that it will also prove to belong to Capnodiales; we therefore suggest that it is listed as “? Capnodiales (incertae sedis)” until fresh data become available.
Racoleus trichophorus R. Sant. & D. Hawksw., sp. nov.
MycoBank MB561240
Fig. 1.
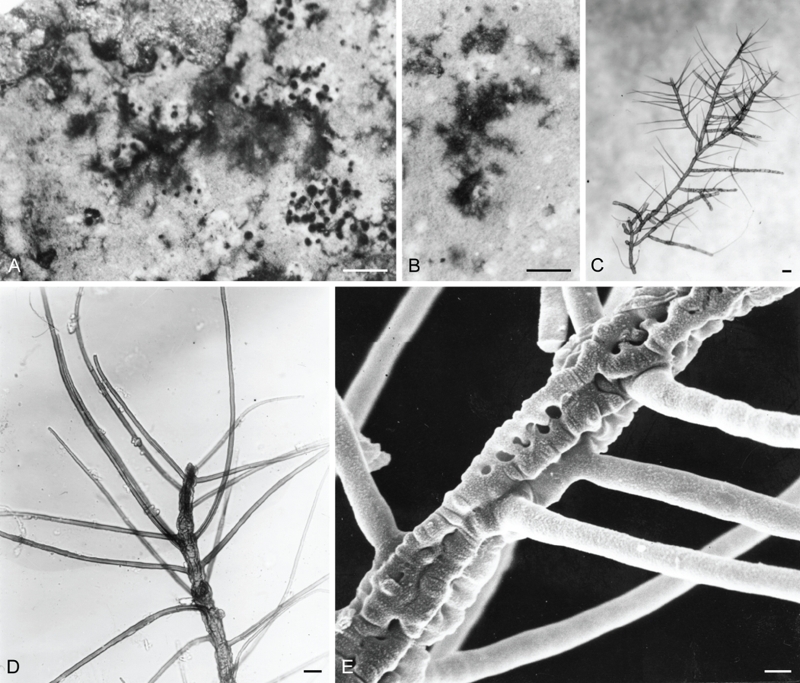
Racoleus trichophorus. A, B. Habit, overgrowing Dichosporidum brunnthaleri on bark. C, D. Detail of lichenized filaments. E. SEM micrograph showing the dentate walls of the hyphae over the algal filament and also the characteristic lateral spines. A-D (Santesson 10344a, UPS – holotype), E. (Santesson P7:4, UPS). Bars A = 225 μm, B = 50 μm, C = 14 μm, D = 7 μm, E = 2 μm.
Fig. 2.
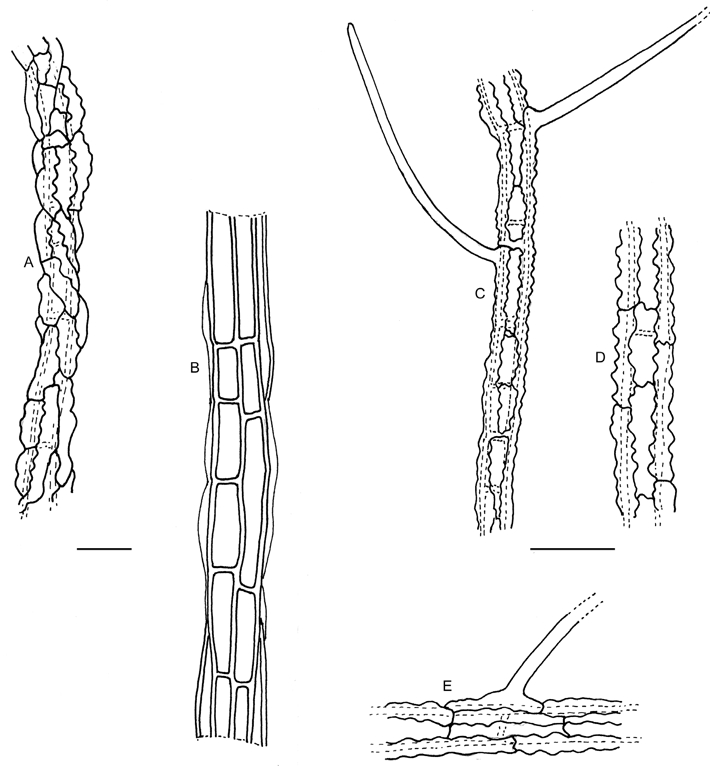
A. Cystocoleus ebeneus filament (Hawksworth, K(M) 82043). B. Racodium rupestre filament (Dalhem, UME 627 10). C-E. Racoleus trichophorus (Santesson, K(M) 165036. C. Filament. D. Detail of dentate hyphal walls. E. Detail of origin of a lateral spine. Bars = 10 μm.
Fig. 3.
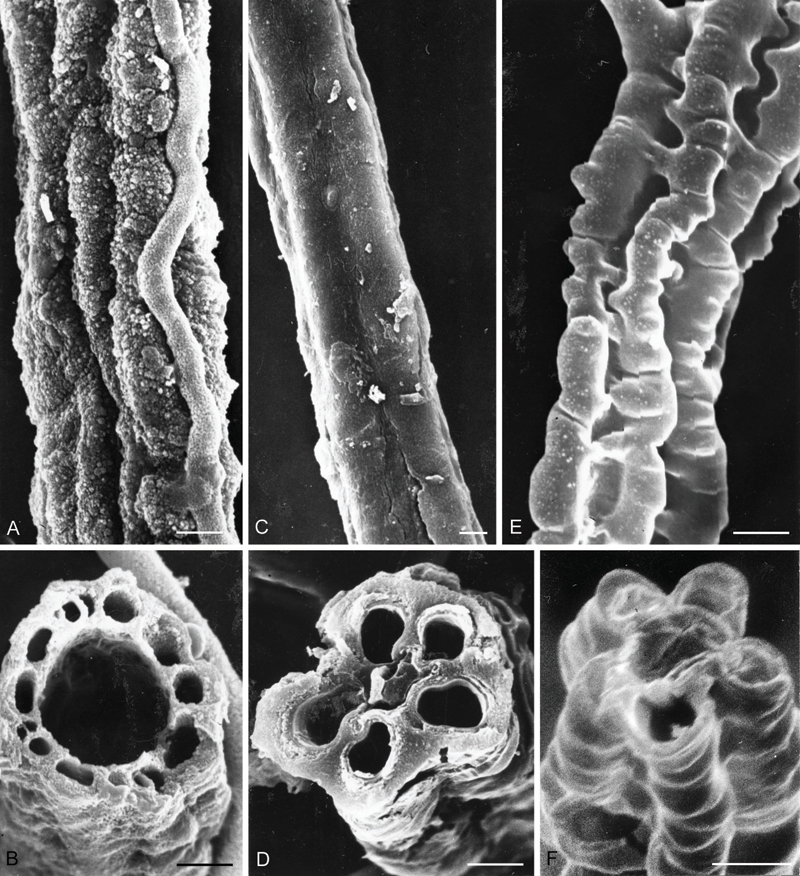
SEM micrographs of filaments. A-B. Cystocoleus ebeneus (Santesson 22339, UPS). C-D. Racodium rupestre (Santesson 14386, UPS). E-F. Racoleus trichophorus (Santesson P7:4, UPS). A, C, and E, Surface views. B, D, and F. Transverse sections. Bars = 2 μm.
Etymology: the epithet recalls the spiny hair like outgrowths.
Thallis lichenibus cum filamentis 7-9 μm latis, et spinis lateralis arcuatis non-lichenibus usque 50–70 x 1.5–3 μm instructis.
Typus: Ivory Coast: Abidjan, in the forest of Banco (ca 5 km north of Abidjan), 5o 30′ N, 4o 0′ W, on trunk of a large tree in a very dark rainforest, overgrowing Dichosporidium brunnthaleri, 29 July 1954, R. Santesson 10344a (UPS- holotypus; GZU - isotypus).
Thallus superficial, forming dense fluffy patches recalling cotton-wool, to 5 mm diam, pale to fuscous brown, filamentous. Photobiont Trentepohlia, single filaments of which are ensheathed by fungal hyphae. Filaments suberect to decumbent or spreading on the surface, sympodially branched, 7–9 μm wide, outer wall undulating and irregularly corrugated, reflecting the morphology of the fungal hyphae, with numerous lateral spines. Hyphae in a single layer surrounding the algal filament, orientated vertically along and always parallel to the axis of the filament, brown, 2–3 μm wide, septate, septa generally 10–15 μm apart, thick-walled, uneven and undulate to corrugated, corrugations tending to interlink with those of adjacent hyphae, not ornamented. Spines arising at broadly acute to almost right angles to the vertical axis, brown, stiff, thick-walled, smooth-walled, not ornamented or corrugated, arcuate to straight, directed outwards and upwards, mainly 50–70 μm in length and 1.5–3 μm wide, gradually tapered towards the tip which is 1–1.5 μm wide, the base expanded into a foot-like cell adhering to the algal filament and measuring 4–7 μm in length. Conidiogenous cells and conidia unknown.
Ecology: All collections are on tree trunks in dense shade in tropical rain forests and on whitish crustose lichens, notably Dichosporidium brunnthaleri, D. nigrocinctum, Pyrgillus indicus, an unidentified arthonioid lichen (probably a species of Cryptothecia). The Racoleus overgrows the crustose lichens and has no intimate contact with them and is easily removed. We do not consider it lichenicolous, and its occurrence on whitish lichens is perhaps a sampling artefact, possibly due to it being more easily visible against a white background.
Distribution: Africa (Ivory Coast), Asia (China), and South America (Peru). The disjunct localities suggest that the species will prove to be pantropical.
Observations: This new genus differs from both Cystocoleus and Racodium in the presence of lateral spines (Fig. 1C–E, 2C, E), as well as in its ecology and distribution. In addition, the hyphae surrounding the algal filament differ in that they are orientated parallel to the filament axis with interlocking corrugations, the surface of the hyphae is smooth in the SEM. In Cystocoleus, similar corrugation occurs but is less pronounced and the hyphae are more irregularly arranged, tending to wrap around the algal filament rather than be strictly orientated along its axis, giving it a more knobbly appearance (Figs 2A, 3A); the surface of the hyphae also appear ornamented in the SEM (Fig. 3A). In Racodium the hyphae lack interlocking corrugations, are thicker-walled than in the other genera, and fused to form elongated rectangular cells orientated vertically along the axis of the algal filament (Fig. 2B) giving an overall smooth rather than a knobbly appearance; the hyphal walls are completely smooth in the SEM (Fig. 3B). The differences between these three genera are summarized in Table 1.
Table 1.
Main anatomical characters distinguishing the genera Cystocoleus, Racodium, and Racoleus.
| Character | Cystocoleus | Racodium | Racoleus |
|---|---|---|---|
| Hyphal arrangement | Twisted | Vertical | Vertical |
| Hyphal wall corrugation | Present | Absent | Present |
| Hyphal wall ornamentation | Warted | Smooth | Smooth |
| Lateral spines | Absent | Absent | Present |
| Distribution | Temperate/Subboreal | Temperate/Subboreal | Tropical |
No description of a fungus recalling Racoleus trichophorus could be found in the lichenological or wider mycological literature we examined. However, as we are less familiar with phycological publications, we cannot totally exclude the possibility that the dual organism has been given a name in an old algological work.
Santesson (1952: 404) had noted that Dodge (1933: 400) mentioned a filamentous lichen with brown hyphae from Costa Rica, and it is conceivable that could have been this species, but no illustration was provided and the material has not been re-examined. Dodge treated this lichen under the name Coenogonium heterotrichum Müll. Arg. (Müller 1893: 162), but as Dodge noted that species has colourless hyphae. This was confirmed in the type material (Costa Rica: San José: San Marcos de Dota, Tonduz, alt. 1200 m, on thallus of Pyxine sp., 1890, Pitt 6115, G-00293681 – holotype) which has algal filaments with some encrusting hyaline hyphae, some of which grow out away from the filaments, but no regular structure is evident with no brown hyphae or any forming jig-saw like patterns on the surface.
Other specimens examined: China: Yunnan Province: Xishuangbanna District, Jinghong Co., Menglun, Electric Station, Monsun forest valley, 21 o 55′ N, 101 o 16′E, alt. ca 500-600 m, on a tree in a rather dark forest in a narrow valley, overgrowing an arthonioid lichen (probably Cryptothecia), 15 Sept. 1987, R. Santesson 32036b (UPS). – Peru: Dept. Loreto: Iquitos, Explorama Lodge (ca 50 km NE of Iquitos), Lake Trail, 3 o 27′ S 72 o 57′ W, alt. ca 100 m, on a tree trunk in a tropical rainforest, overgrowing Dichosporidium nigrocinctum, 23 Jan. 1981, R. & B. Santesson P7:4 (K(M) 165036, S, UPS); Dept. San Martin: Prov. Lamas, Cerro Blanco (ca 63 km on road W-WNW of Tarapoto), ca 6 o 25′ S 76 o 40′ W, alt. ca 1200 m, on a tree trunk in a dark rainforest, overgrowing Pyrgillus indicus, 17 Mar. 1981, R. Santesson & G. Thor P77:11 (S, UPS).
Cystocoleus Thwaites, Ann. Mag. nat. Hist., ser. 2 3: 241 (1849).
Type: Cystocoleus ebeneus (Dillw.) Thwaites 1849.
Cystocoleus ebeneus (Dillw.) Thwaites, Ann. Mag. nat. Hist., ser. 2 3: 241 (1849).
Basionym: Conferva ebenea Dillw., Br. Confervæ: pl. 101 (1809).
Synonyms: Croolepus ebeneus (Dillw.) C. Agardh, Syst. Alg.: 36 (1824).
Racodium ebeneum (Dillw.) Fr., Summa Veg. Scand. 1: 122 (1846).
Coenogonium ebeneum (Dillw.) A.L. Sm., Mongr. Br. Lich. 2: 3 (1911).
Coenogonium germanicum Glück, Flora 82: 268 (1896).
Coenogonium schmidlei Simmer, Allgem. Bot. Zeit. 5: 190 (1899).
Byssus nigra auct. p. p., non Huds., Fl. Anglica: 487 (1762).
Conferva nigra auct. p. p., non (Huds.) Roth, Catal. Bot. 3: 299 (1805).
Cystocoleus nigra auct. p. p., non (Huds.) Hariot, J. Bot. 4: 91 (1890); as “niger”.
Coenogonium nigrum auct. p. p., non (Huds.) Zahlbr., Ann. Naturhist. Mus. Wien 25: 241 (1911).
Type: United Kingdom: Morayshire: “On the stump of a tree [sic!] in Macbeth’s Wood [Brodie] nr Forres”, Aug. 1807, W. J. Hooker (BM – lectotypus hic designatus).
Descriptions and illustrations (selected): Glück (1896), Jørgensen (1986), Schade (1932), Skuja & Ore (1935), Smith (1926), Smith et al. (2009), and Wirth (1995).
Exsiccatae: Anzi, Lich. Langob. no. 495 (BM, UPS); Krypt. Exs. Vindob. no. 1638 (BM, UPS): Mougeot & Nestler, Stirps Crypt. no. 400 (BM, UPS; with Racodium rupestre); Rabenhorst, Lich. Eur. no. 841 (BM, UPS; with Racodium rupestre); and Räsänen, Lichenotheca Fenn. no. 360 (BM, UPS).
Number of species: Monotypic.
Ecology: On vertical or somewhat inclined or underhanging siliceous rocks out of direct rain, but also on soil or eroded moss cushions in the subantarctic islands (Jørgensen 1986). The species often grows mixed with Racodium rupestre. Ecological, including quadrat, data are provided by several authors, including James et al. (1977), Schade (1932), and Wirth (1972). The communities formed, black felt-like patches over extensive areas of rock, are so conspicuous that they have been given the phytosociological name Racodietum rupestris Schade 1924. The most commonly associated lichens are species of Lepraria and Leproloma.
Distribution: Europe (partly mapped by Wirth 1972), North America (Canada, USA), South America (Argentina, Bolivia, Chile, Colombia, Peru), Africa (Kenya), Asia (Mongolia), Australasia (New Zealand, Tasmania), and Antarctica (subantarctic islands).
Observations: We wish to draw attention to the overlooked and painstaking work on the culture of this fungus reported by Skuja & Ore (1935) and illustrated by colour plates. After two months in pure water, fungal hyphae grew free from the algal filaments, spreading irregularly, branching, and retaining a nodulose appearance (loc. cit.: table I fig. 4), quite unlike the lateral hairs in Racoleus. These workers also found that the Trentepohlia also grew out separately when cultured on Beneckeís agar (loc. cit.: table I figs 5-8). In fresh material in the field, tufts of hyphae similar to those reported by Skuja & Ore are occasionally encountered.
There is also an interesting observation recorded in an annotation by W Watson (1872-1960) on a mixed collection with Racodium rupestre from near Shepley in BM that “When treated with strong nitric acid the Coenogonium [i.e. Cystocoleus] appears reddish with the filament hyphae twisted, whilst the Racodium remains dark with hyphae parallel”.
Øvstedal & Smith (2001) comment that: “The Antarctic populations differ somewhat from the North European ones and may be an undescribed taxon” but do not elaborate further, and no divergences were noted by Lindsay (1971). Material from Antarctica was not included in the molecular study of Muggia et al. (2008).
Glück (1896), in a critical but little-cited study, distin-guished Coenogonium germanicum from Cystocoleus rupestris on the basis of the differences in the arrangement of the hyphae which he also illustrated in transverse sections. He cultured the algal partners, which he referred to different species of Trentepohlia. However, Glück’s critically executed illustrations leave no doubt as to the application of his species name; one of these could be designated as lectotype (e.g. Pl. 7 figs 1–5) if no original material can be located in HEID or M where specimens could be located. Simmer (1899) illustrated Coenogonium germanicum and compared it with his newly described C. schmidlei; both had the irregular hyphal arrangements typical of Cystocoleus ebeneus, and he seems to have separated them because of the proliferation of non-lichenized hyphae in Coenogonium germanicum. Original material of C. schmidlei, ex-herb. Reimers in B, examined by R.S. and L.T. is indeed Cystocoleus ebeneus, so confirming the synonymy.
Lindau (1913, 1923) used Glück’s name in the sense of Cystocoelus ebeneus, according to his description, and Zahlbruckner (1924) listed it as a synonym of the present species.
Lindsay (1971) commented that Coenogonium kerguelense, described by Dodge (1966) from Kerguelen Island, could either be a Coenogonium or the Cystocoleus. Through the courtesy of Alan Fryday, D.L.H. was able to examine photomicrographs of one of the two syntypes (Cote 1000, tapissant les alveoles de la face N.E. du Mont Campbell, 6 Nov. 1952, Albert de la Rue # 64) in FH. This comprised a creamy yellowish buff felted colony which microscopically comprised algal filaments ca 20 μm wide with an encrusted surface. No distinctive Cystocoleus or other dematiaceous hyphae were apparent in the photomicrographs, and the width of the filaments is outside the normal 19-15 μm range of C. ebeneus. Dodge’s name consequently appears to be based on a colony of a non-lichenized Trentephohlia species.
Nomenclature: The name Byssus nigra is of uncertain application and never appears to have been formally typified, but some authors have used the epithet for Cystocoleus ebeneus in the past. The name was introduced by Hudson (1762) for a stiff filamentous organism found on calcareous rocks near Ingleborough and Settle in Yorkshire; a most improbable habitat for this lichen. However, he subsequently amended the notes on ecology and distribution to “in rupibus et saxis grandioribus in boreali parte Angliae et in Wallia” and referred to it as the “Anglis black byssus” (Hudson 1778). Byssus nigra was featured as “Black rock byssus” in English Botany (Smith & Sowerby 1800), with a reference back to Hudson (1778), but not Hudson (1762) from the page number cited (i.e. p. 606 and not 487), and some earlier usages and polynomials. The original material on which Sowerby’s illustration was based could not be located in BM, but an index to the original plates and specimens held there gives the modern name as Racodium rupestre and not Cystocoleus ebeneus or any of its synonyms.
Hudson’s binominal was listed as a synonym of Conferva ebenea by Dillwyn (1809) when introducing that name, but, interestingly from his citing “p. 606” he was also referring to the second edition of Hudson’s work as had Smith – who is not mentioned by Dillwyn. Smith’s text indicates that it is most likely he was dealing with either C. ebeneus or Racodium rupestre from his description of the habitat and comment that “it is always found on a micaeous or quartzose stone”. It appears to have been applied to both species by early authors and so is best listed as a “pro parte” usage under each pending any formal typification.
A complication in the nomenclatural situation arises from the existence of a nomenclaturally independent name Conferva nigra Huds. 1762 based on a different type – indeed, that taxon was described as abundant on the seashore in Yorkshire. Dillwyn (1809) indicated that he had seen authentic specimens of that taxon, and considered to represent a seaweed which he called Conferva atro-rubescens Dillw. 1809 – a red alga for which the current name is Polysiphonia nigra (Huds.) Batters 1902, following neotypification of this binominal of Hudson’s by Maggs & Hommersand (1993). The existence of this name means that Dillwyn’s citing of Byssus nigra as a synonym does not render Conferva ebenea as superfluous and illegitimate under Art. 52.1 as a combination of Byssus nigra into Conferva would have created a homonym to be rejected under Art. 53.1.
In introducing the generic name Cystocoleus, “with the sanction of my friend, the Rev. M. J. Berkeley”, Thwaites (1849) listed “Byssus nigra, E.B. t. 702!” as a synonym. However, as he did not refer to Hudson at all and it is clear that he was listing the English Botany usage (see above), rather than treating the name as a synonym. In this, Thwaites was perhaps following Hooker (1844: 385) who did not mention Hudson and attributed the binominal Byssus nigra only to “Sm.” in his index (Hooker 1844: 422). The legitimacy of Thwaites’ combination is not therefore threatened by Hudson’s name as he did not treat it as a synonym, merely listing the usage in English Botany.
We see no nomenclatural obstacle to the continued use of the name Cystocoleus ebeneus. If Hudson’s original material or a later typification of his name were discovered, and that indeed proved to belong to this taxon or to Racodium rupestre, the epithet should be proposed for rejection in order to maintain whichever of the two currently used names was threatened.
Typification: In the original account of Dillwyn (1809), three specimens were mentioned: (1) “Dillenius. – On Rocks in the Highlands. James Brodie, Esq.”; (2) On the stump of a dead tree in Mackbeth’s Wood, at Brodie, near Forres, N.B. W.J. Hooker, Esq.”; and (3) “On Birch trees, at Coftefy near Norwich. Mr. S. Wilkins.” The second collection is present in BM (labelled “On the stump of a tree in Macbeth’s Wood nr Forries [sic!], Aug. 1807”), and despite the unusual habitat reported it is an appropriate lectotype for Dillwyn’s name. However, the specimen is not on a piece of bark or wood, does not have any evident adhering woody fragments, but does have some granitic crystals intermixed; this causes us to doubt that it was growing directly on a tree-stump. That Thwaite’s (1849: pl. 8 figs 1–3) actually illustrated a specimen of Racodium rupestre does not affect the application of the generic name as “Conferva ebenea, Dillw. t. 101” was given as the basionym of Cystocoleus ebeneus.
Racodium Fr., Syst. Mycol. 3: 229 (1829); nom. cons.
Synonyms: Rhacodium Spreng., Linn. Syst. Veg., 16th edn, 4: 557 (1827); orth. var., nom. illegit. (Arts. 52.1, 60.1)
Rhacodiopsis Donk, Persoonia 8: 276 (1975); nom. illegit. (Art. 52.1).
Non Racodium Pers., Neues Mag. Bot. 1: 123 (1794) : Fr., Syst. Mycol. 1: xlvi (1821); nom. rej.
Type: Racodium rupestre Pers. 1794.
Racodium rupestre Pers., Neues Mag. Bot. 1: 123 (1794).
Synonyms: Byssus rupestris (Pers.) DC., in Lamarck & DeCandolle, Fl. Franç. 3rd edn 2: 592 (1805).
Dematium rupestre (Pers.) Nees, Syst. Pilze: 76 (1816).
Cystocoleus rupestris (Pers.) Rabenh., Krypt.-Fl. Sachsen 2: 75 (1870).
Rhacodiopsis rupestris (Pers.) Donk, Persoonia 8: 276 (1975).
Byssus nigra auct. p. p., non Huds., Fl. Anglica: 487 (1762).
Conferva nigra auct. p. p., non (Huds.) Roth, Catal. Bot. 3: 299 (1805).
Cystocoleus nigra auct. p. p., non (Huds.) Hariot, J. Bot. 4: 91 (1890); as “niger”.
Coenogonium nigrum auct. p. p., non (Huds.) Zahlbr., Ann. Naturhist. Mus. Wien 25: 241 (1911).
Type: United Kingdom: Wales: “Racodium rupestre P. Byssus nigra Engl. Bot. Wales. Hb. Pers” (L 910.263-1045 pro parte – lectotypus hic designatus).
Descriptions and illustrations (selected): Brodo et al. (2001), Glück (1896), Smith (1926), Smith et al. (2009), Thwaite’s (1849, as Cystocoelus ebeneus).
Exsiccatae: De Thümen, Mycotheca Univ. no. 198 (BM, UPS); Kunze & Lahm, Myc. Exs. no. 25 (BM); Mougeot & Nestler, Stirps Crypt. no. 400 (BM, UPS; with Cystocoleus ebeneus); Rabenhorst, Lich. Eur. no. 841 (BM, UPS; with C. ebeneus); Moberg, Lich. Sel. Upsal. no. 45 (BM, UPS); Tobolewski, Lichenotheca Polon. fasc. 3 no. 6 (BM): Vě zda, Lich. Sel. Exs. no. 450 (BM, UPS).
Number of species: Monotypic.
Ecology: As for Cystocoleus ebeneus.
Distribution: Europe (most countries), North America (Canada, USA), South America (Argentina, Chile), Africa (South Africa), Asia (Japan), and Australia (Tasmania).
Observations: Zahlbruckner (1905, 1926), Vainio (1921), Dodge (1933) and Christiansen (1947) mention the alga in Racodium as belonging to the genus Cladophora rather than to Trentepohlia, but the basis for this is obscure. In order to resolve this matter, the alga was isolated into pure culture by Koch (1962) who found it to be a member of the Trentepohlia aurea group.
Nomenclature: The nomenclatural issues surrounding this name are complex. Riedl (1968) and Hawksworth (1970) independently noted that “Racodium” was being used for a sterile filamentous lichen by lichenologists, and for a quite different non-lichenized hyphomycete by mycologists. These authors both concluded that the name should be typified by the lichenized element (i.e. R. rupestre) rather than the conidial fungus (i.e. R. cellare Pers. 1794), but in a posthumous publication Donk (1975) disagreed. In order to resolve the matter, Hawksworth & Riedl (1977) proposed the name for conservation for the lichenized fungus, their proposal was accepted and it is now list as conserved in the Code. The name Rhinocladiella ellisii D. Hawksw. 1977 was introduced for the conidial state of the “cellar fungus”. However, sterile material of the same species, which does not form conidia, had been referred to as Zasmidium cellare (Pers.) Fr. 1829 and was considered a synanamorph. De Hoog (1979) did not consider these names as synanamorphs as the conidiogenous cells were micronematous and not markedly different from the sterile hyphae and consequently commend the use of Zasmidium cellare for this fungus. This interpretation has been followed in the molecular phylogenetic study of Arzanlou et al. (2007), who found that it clustered with Ramichloridum species and published photographs of the conidiogenous cells and conidia.
Donk (1975) noted that “Rhacodium” was orthographically the more correct spelling of the generic name, as derived from the Greek “ραXoς” (rag), but the form “Racodium” is that conserved in accordance with general usage.
Vainio (1921: 238) listed Coenogonium germanicum as a synonym of Racodium rupestre, but the original illustrations of Glück (1896) and those of Simmer (1899) are of Cystocoleus ebeneus as interpreted here.
Henssen & Jahns (1973) evidently regarded this species as congeneric with Cystocoleus, but did not explain why; molecular data show that view to be unsupportable.
Typification: The typification of the name Racodium rupestre has not been addressed and a formal typification published. In order to clarify the issue, the specimens in Persoon’s collection in L were studied by R.S. in 1956 who did not publish his results at that time. R.S. found that there were four specimens under this name:
L 910.264-801: “210 Racodium rupestre Pers. Syn. in rupibus umbrosis Moug. in hb. Pers.”. This is Cystocoleus ebeneus (with sparse R. rupestre).
L 910.263-1045: “Racodium rupestre P. Byssus nigra Engl. Bot. Wales. Hb. Pers.” This is R. rupestre (with sparse C. ebeneus).
L 910.264-922: “Racodium rupestre Pers. Hb. Pers.”. This is C. ebeneus with no Racodium.
L 910-264-701: “No. 75. An Sandhalen. M. Aug. Racodium rupestre. Hb. Pers.” This consists only of non-lichenized fungal hyphae.
Three of the collections were mixed with Cystocoleus ebeneus, and it is evident that in practice Persoon applied Racodium rupestre in a sense embracing both genera. This is hardly surprising as the species commonly grow mixed together and require microscopic study to separate them with confidence. In order to fix the application of Persoon’s name in the sense in which it is currently used, L 910.263-1045 is consequently designated as lectotype here as that is the only one in which the Racodium predominates.
Acknowledgments
We are indebted to the curators of all collections cited in the text and others from which we have examined material during this study for access to collections in their care (B, BM, G, K, L, MAF, UME, UPS, and S), Göran Thor (Swedish Agricultural University, Uppsala) for identification of the lichens being overgrown by the Racoleus, and Alan M. Fryday (Michigan State University) who kindly made available photomicrographs of Coenogonium kerguelense. Work on the text and the drawings included in this paper commenced while D L.H. was a visiting researcher at the University of Umeå in 1999, and he is grateful to Ove E. Eriksson for the facilities provided at that time. It was completed while D.L.H. was in receipt of support from the Ministerio de Educación y Ciencia of Spain (Proyectos I+D CGL 2008-01600).
REFERENCES
- Arzanlou M, Groenewald JZ, Gams W, Braun U, Crous PW. (2007) Phylogenetic and morphotaxonomic revision of Ramichloridium and allied genera. Studies in Mycology 58: 57–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodo IM, Sharnoff SD, Sharnoff S. (2001) Lichens of North America. New Haven: Yale University Press; [Google Scholar]
- Christiansen MS. (1947) Bidrag til Danmarks lavflora. I. Botanisk Tidsskrift 48: 172–191 [Google Scholar]
- Crous PW, Schoch CL, Hyde KD, Wood AR, Gueidan C, de Hooog GS, Groenewald JZ. (2009) Phylogenetic lineages in the Capnodiales. Studies in Mycology 64: 17–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubero OF, Crespo A, Fatehi J, Bridge PD. (1999) DNA extraction and PCR amplification method suitable for fresh, herbarium stored and lichenized fungi. Plant Systematics and Evolution 217: 234–249 [Google Scholar]
- David JS, Rands DG, Lachapelle M. (1989) Heavily lichenized Physolinum (Chlorophyta) from a dimly lit cave in Missouri. Jourmal of Phycology 25: 419–428 [Google Scholar]
- Dillwyn LW. (1809) British Confervæ; or coloured figures and descriptions of the British plants referred by botanists to the genus Conferva. London: W Phillips; [Google Scholar]
- Dodge CW. (1933) The foliose and fruticose lichens of Costa Rica. I. Annals of the Missouri Botanical Garden 20: 373–467 [Google Scholar]
- Dodge CW. (1966) Lichens from Kerguelen collected by E. Aubert de la Rüe. Comité National Français des Recherches Antarctique 15: 1–8 [Google Scholar]
- Donk MA. (1975) Racodium Pers. not a genus of lichens. Persoonia 8: 273–276 [Google Scholar]
- Glück H. (1896) Eine deutisches Coenogonium. Flora 82: 269–285 [Google Scholar]
- Hawksworth DL. (1970) A nomenclatural note on Racodium Pers. Transactions of the British Mycological Society 54: 323–326 [Google Scholar]
- Hawksworth DL, Riedl H. (1977) Proposal to conserve the generic name Racodium Fr. Taxon 26: 208 [Google Scholar]
- Henssen A, Jahns HM. (1973) [“1974. ”] Lichenes: Eine Einführung in die Flechtenkunde. Stuttgart: G Thieme Verlag; [Google Scholar]
- Hoog GS de. (1979) Nomenclatural notes on some black yeast-like hyphomycetes. Taxon 28: 347–348 [Google Scholar]
- Hooker WJ. (1844) The English Flora of Sir James Edward Smith. Vol. 5 (1). Classis XXIV. Cryptogamia. London: Longman, Brown, Green and Longmans; [Google Scholar]
- Hudson W. (1762) Flora Anglica. London: J Nourse and C Moran; [Google Scholar]
- Hudson W. (1778) Flora Anglica. 2nd edn London: J Nourse; [Google Scholar]
- James PW, Hawksworth DL, Rose F. (1977) Lichen communities in the British Isles: a preliminary conspectus. In: Lichen Ecology (Seaward MRD, ed.): 295–413 London: Academic Press; [Google Scholar]
- Jørgensen PM. (1986) Macrolichens of Bouvetøya. Norsk Polarinstitut Skrifter 185: 23–34 [Google Scholar]
- Koch W. (1962) Die Gonidie von Racodium rupestre Pers. Vorträge aus dem Gesamtgebiet der Botanik, neue folge 1: 61–64 [Google Scholar]
- Lindau G. (1913) Die Flechten. [Kryptogamenflora för Anfänger no 3.] Berlin: Julius Springer; [Google Scholar]
- Lindau G. (1923) Die Flechten. 2nd edn [Kryptogamenflora för Anfänger no 3.] Berlin: Julius Springer; [Google Scholar]
- Lindsay DC. (1971) Notes on Antarctic lichens: III. Cystocoleus niger (Huds.) Hariot. British Antarctic Survey Bulletin 24: 119–120 [Google Scholar]
- Maggs CA, Hommersand MH. (1993) Seaweeds of the British Isles. Vol.1. Rhodophyta. Part 3A. Ceramiales. London: Her Majesty’s Stationery Office; [Google Scholar]
- Muggia L, Hafellner J, Wirtz N, Hawksworth DL, Grube M. (2008) The sterile microfilamentous lichenized fungi Cystocoleus ebeneus and Racodium rupestre are relatives of plant pathogens and clinically important dothidealean fungi. Mycological Research 112: 50–56 [DOI] [PubMed] [Google Scholar]
- Müller J. (1893) [Primitiae Florae Costaricensis.] Lichenes, seconde énumeration. Bulletin de la Société Royale de Botanique de Belgique 32: 122–173 [Google Scholar]
- Øvstedal DO, Smith RIL. (2001) Lichens of Antarctica and South Georgia: a guide to their identification and ecology. Cambridge: Cambridge University Press; [Google Scholar]
- Riedl H. (1968) Die Gattungsnamen Racodium Persoon und Zasmidium Fries. Taxon 17: 34–37 [Google Scholar]
- Ruibal C, Gueidan C, Selbmann L, Gorbushina AA, Crous PW, Groenewald JZ, Muggia L, Grube M, Isola D, Schoch CL, Staley JT, Lutzoni F, de Hoog GS. (2009) Phylogeny of rock-inhabiting fungi related to Dothideomycetes. Studies in Mycology 64: 123–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santesson R. (1952) Foliicolous lichens I. A revision of the taxonomy of the obligately foliicolous, lichenized fungi. Symbolae Botanicae Upsaliensis 12( 1): 1–590 [Google Scholar]
- Schade [F]A. (1932) Die Verbrietung von Racodium rupestre Pers. und Coenogonium nigrum (Huds.) Zahlbr. in Sachsen nebst einigen biologischen Bemerkungen. Beihefte zur Botanisches Zentralblatt 49 (Erganzungsband): 421–437 [Google Scholar]
- Simmer H. (1899) Dritter Bericht über die Kryptogamenflora der Kreuzeck-gruppe in Kärten. Allgemeine Botanische Zeitschrift 5: 189–194 [Google Scholar]
- Skuja H, Ore M. (1935) Die Flechte Coenogonium nigrum (Huds.) Zahlbr. und ihre Gonidie. Acta Horti Botanici Universitatis Latviensis 8: 21–44 [Google Scholar]
- Smith AL. (1926) A Monograph of British Lichens. Vol. 2 2nd edn London: British Museum (Natural History) [Google Scholar]
- Smith CW, Aptroot A, Coppins BJ, Fletcher A, Gilbert OL, James PW, Wolseley PW. (2009) The Lichens of Great Britain and Ireland. London: British Lichen Society; [Google Scholar]
- Smith JE, Sowerby J. (1799–1800) English Botany. Vol. 10 London: J Sowerby; [Google Scholar]
- Thwaites GHK. (1849) Note on Cystocoleus, a new genus of minute plants. Annals and Magazine of Natural History, series 2, 3: 241–242 [Google Scholar]
- Vainio EA. (1921) Lichenographia Fennica I. Pyrenolichenes usque proximi Pyrenomycetes et Lichenes Imperfecti. Acta Societatis pro Fauna et Flora Fennica 49(2): 1–274 [Google Scholar]
- Wirth V. (1972) Die Silikatflechten-Gemenschaften im außeralpinum Zentraleuropa. Dissertationes Botanicae 17: 1–306, 1–9 [Google Scholar]
- Wirth V. (1995) Die Flechten Baden-Württembergs. 2nd edn Vol. 1 Stuttgart: Eugen Ulmer; [Google Scholar]
- Zahlbruckner A. (1903-08) [Lichenes (Flechten)] B. Specieller Teil. Die natürlichen Pflanzenfamilien 1 (1*): 49–349 [Google Scholar]
- Zahlbrucker A. (1923-24) Catalogus Lichenum Germaniae. Vol. 2 Leipzig: G Borntraeger; [Google Scholar]
- Zahlbruckner A. (1926) [Lichenes (Flechten)] B. Spezieller Teil. Die natürlichen Pflanzenfamilien [ 2nd edn] 8: 61–270 [Google Scholar]


