Upregulation of γ-secretase or overexpression of presenilin 1, a subunit of the γ-secretase complex, in cultured endothelium and in the mouse eye suppressed both in vitro and in vivo angiogenesis. This was associated with a reduction in angiogenic factors, oxidative stress and local inflammation.
Abstract
Purpose.
This study aimed to determine whether upregulation of γ-secretase could inhibit laser-induced choroidal neovascularization (CNV) and if this was associated with a reduction in both oxidative stress and proinflammatory cytokines.
Methods.
γ-Secretase, or its catalytic subunit presenilin 1 (PS1), were upregulated by exposure to either pigment epithelial derived factor (PEDF) or an AAV2 vector containing a PS1 gene driven by a vascular endothelial-cadherin promoter. Retinal endothelial cells were infected with AAV2 or exposed to PEDF in the presence or absence of VEGF and in vitro angiogenesis determined. Mouse eyes either received intravitreal injection of PEDF, DAPT (a γ-secretase inhibitor) or PEDF + DAPT at the time of laser injury, or AAV2 infection 3 weeks before receiving laser burns. Lesion volume was determined 14 days post laser injury. Superoxide generation, antioxidant activity and the production of proinflammatory mediators were assessed. Knockdown of γ-secretase was achieved using siRNA.
Results.
γ-Secretase upregulation and PS1 overexpression suppressed VEGF-induced in vitro angiogenesis and in vivo laser-induced CNV. This was associated with a reduction in the expression of VEGF and angiogenin 1 together with reduced superoxide anion generation and an increase in MnSOD compared with untreated CNV eyes. PS1 overexpression reduced proinflammatory factors and microglial activation in eyes with CNV compared with control. siRNA inhibition of γ-secretase resulted in increased angiogenesis.
Conclusions.
γ-Secretase, and in particular PS1 alone, are potent regulators of angiogenesis and this is due in part to stabilizing endogenous superoxide generation and reducing proinflammatory cytokine expression during CNV.
Neovascularization is a major cause of vision loss in patients with age-related macular degeneration (AMD) that is characterized by abnormal, new vessel growth into the subretinal space from the underlying choroid resulting in choroidal neovascularization (CNV).1,2 It is now quite evident that there is a plethora of pro- and anti-angiogenic factors that regulate the ocular vasculature and are involved in the development and progression of aberrant neovascularization such as AMD. The collective evidence suggests that the VEGF family is critical for ocular angiogenesis and treatment of AMD patients with CNV with agents that neutralize extracellular VEGF significantly reduce CNV.3,4 Pigment epithelium-derived factor (PEDF) is a potent inhibitor of VEGF-induced angiogenesis5,6 and the RPE is a major source of PEDF in the retina. The decline in expression of PEDF, both with increasing age and in AMD, facilitates a proangiogenic subretinal environment.7–9 Although numerous studies have demonstrated that PEDF inhibits VEGF-induced CNV the mechanisms are poorly understood and no definitive receptor has yet been identified. Previously, we have reported that PEDF inhibits VEGF-induced angiogenesis in cultured microvascular endothelial cells. This inhibition is due to a γ-secretase dependent cleavage and intracellular translocation of VEGF receptor (VEGFR) 1 10 and others have reported that VEGFR2 is cleaved in a similar fashion in RPE cells.11
γ-Secretase is a complex composed of four different integral membrane proteins: presenilin (PS), nicastrin, Aph-1, and Pen-2.12–14 The most studied component of the γ-secretase complex is presenilin which is an integral enzyme in the cleavage of amyloid precursor protein and contributes to the accumulation of amyloid-β peptide in Alzheimer's disease. Activation of PS is dependent on its endoproteolysis of full length PS into an N-terminal fragment (NTF) and C-terminal fragment (CTF).10,12 Nicastrin appears to be necessary for substrate recognition by the γ-secretase complex and nicastrin binding to the substrate is required before presenilin can exert its proteolytic activity.15 Of the two remaining proteins, which constitute γ-secretase, Aph-1 is believed to be a scaffolding protein and Pen-2 appears to regulate PS activity. Assembly of the γ-secretase complex begins in the endoplasmic reticulum and is concluded after translocation of the four proteins to the cell membrane.12,14 Valine residue(s) followed with charged residues within the transmembrane domain serve as cleavage sites for γ-secretase16 and we have recently demonstrated that valine 767 is critical for VEGFR1 cleavage by γ-secretase.17 It is also evident that PS can regulate protein trafficking and protein-protein interactions independently of its protease activity and association with the γ-secretase complex.17–20
There is extensive evidence that oxidative stress is associated with both the wet and dry forms of AMD.1,2,21 Antioxidants such as N-acetyl-cysteine and siRNA against p22phox (an integral subunit of NAPDH oxidase) are potent inhibitors of laser-induced CNV in animal models.22,23 Furthermore, a combination of oxidative stress and vascular growth can lead to an upregulation of proinflammatory cytokines which further exacerbate the progression of CNV24 and anti-inflammatory strategies have been shown to ameliorate CNV.25–27 PEDF, which we have shown regulates γ-secretase activity in cultured cells,10,17 has been previously reported to reduce both oxidative stress and inflammation in the retina.28,29 We therefore decided to determine in this study whether increasing expression of the γ-secretase complex, or of PS alone, can inhibit laser-induced CNV in the mouse CNV model and if this is associated with protection of the retina from oxidative damage and inflammation.
Materials and Methods
Materials
Agglutinin I rhodamine labeled Ricinus Communis was purchased from Vector Laboratories, Inc. (Burlingame, CA). Dihydroethidium was obtained from Invitrogen (Carlsbad, CA). Recombinant VEGF165 was purchased from R&D Systems (Minneapolis, MN) and PEDF from BioProducts MD (Middletown, MD). γ-Secretase inhibitors L685485, DAPT and antibody to mouse β-actin were obtained from Sigma (St. Louis, MO). Antibodies to angiogenin 1 and to VEGF-A were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies against HA-tag, presenilin1, NFκB, and F4/80 were from Abcam (Cambridge, MA), and Anti-Iba1 was from Wako Chemicals USA, Inc. (Richmond, VA). siRNA for PS1, NCT, Aph-1, and Pen-2 was obtained from Applied Biosystems (Carlsbad, CA).
AAV Vectors
A recombinant AAV serotype 2 quadruple tyrosine to phenylalanine (Y-F) capsid mutant (Y444F, Y500F, Y700F, and Y704F) containing the vascular endothelial cadherin (VEC) promoter (kindly provided by Jeremy Nathans, Johns Hopkins University) driving mouse PS1 cDNA tagged with human influenza hemagglutinin (HA) was generated (Fig. 1A). An unmodified capsid AAV2 without PS1 gene and HA tag served as a control. AAV vectors were packaged and purified as previously described.30 Vectors were then resuspended in balanced salt solution with 0.014% Tween-20, titered by quantitative real-time PCR (qRT-PCR) and stored at −80°C.
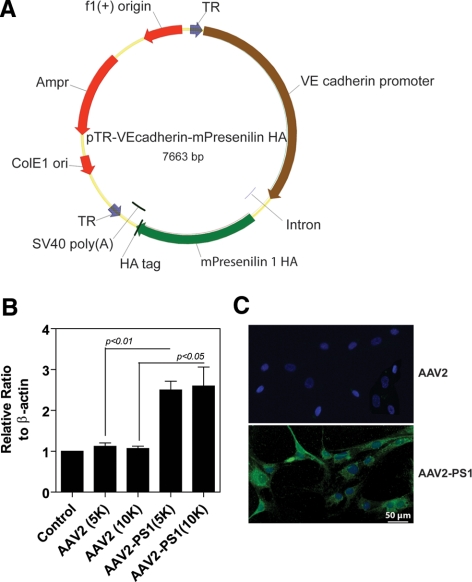
Figure 1.
Vector map and AAV-mediated overexpression of PS1 in cultured microvascular endothelial cells. (A) Vector map depicting the mouse PS-1 gene driven by the VE-cadherin promoter tagged with a human influenza hemagglutinin (HA) reporter gene that was inserted into a recombinant quadruplet mutant AAV2 referred to as AAV2 quadYF-PS1. Control AAV2 without the PS1 gene and HA tag served as a control. (B) Representative Western blot analysis demonstrating PS1 overexpression 48 hours after infection of cells with AAV2 quadYF-PS1 at a multiplicity of infection (MOI) of 5000 or 10,000. Untreated cells and cells infected with control AAV2 acted as controls. The bar graph represents quantitative analysis of PS1 expression normalized against β-actin (mean ± SEM, n = 3). (C) Representative fluorescence micrographs of endothelial cells 48 hours after infection with AAV2 quadYF-PS1 or control AAV2 immunostained with an antibody against HA-FITC. The majority of endothelial cells infected with AAV2 quadYF-PS1 exhibited positive HA staining while no apparent fluorescence was observed in cells infected with control AAV2.
Vector Transfection and Tube Formation Assay in Cell Culture
Bovine retinal microvascular endothelial cells were isolated and grown in microvascular endothelia cell basal medium (MCDB 131) media (Invitrogen) as described previously.31 The early passages (P1-P2) were used for AAV transgene detection and the in vitro angiogenesis tube formation study. After cells were approximately 80% confluent, AAV2-PS1 or wild type AAV2 were diluted in MCDB 131 medium to infect the cells at a MOI of 5000 to 10,000. Twenty-four hours post viral infection, the cells were treated with medium containing PEDF (100 ng/mL) in the presence or absence of VEGF (100 ng/mL) overnight at 37°C in 5% CO2.
The tubule formation assay was undertaken as previously described.10 In brief, near confluent endothelial cells (infected and noninfected) pretreated with growth factors or medium alone for 48 hours were detached and plated sparsely (2.5 × 104 per well) on 24-well plates coated with 12.5% (vol/vol) basement membrane matrix (Matrigel; BD Biosciences, Bedford, MA) and left overnight. The medium was then aspirated, and 250 μL per well of 12.5% basement membrane matrix (Matrigel) was overlaid on the cells for 2 hours to allow polymerization of the basement membrane matrix (Matrigel), followed by adding 500 μL per well of MCDB 131 medium for 24 hours. Culture plates were observed under a phase contrast microscope and photographed at random in five different fields (magnification, × 10). The tubule length (mm/mm2) was quantified.
In Vitro Detection of Transgene and Western Blot Analysis
Endothelial cells were harvested 48 hours post viral infection and fixed in 4% paraformaldehyde and permeabilized with 0.3% Triton X-100. Nonspecific staining was blocked with 5% BSA in PBS for 30 minutes at room temperature. The cells were stained with polyclonal anti-HA tag conjugated FITC all at 1:500 in PBS containing 1% BSA at 4°C overnight. Cells were then rinsed and observed by fluorescence microscopy. For Western blot analysis, 30 μg of cell lysate was separated by electrophoresis on a 7.5%–12% SDS-polyacrylamide gel and proteins transferred onto nitrocellulose membranes (Bio-Rad, Hercules, CA) and nonspecific binding sites blocked with 10% nonfat dry milk. Blots were incubated with a polyclonal antibody against presenilin 1 and, for a loading control, a monoclonal anti-β-actin antibody followed by an HRP-conjugated secondary antibody. The membranes were incubated with a Western blotting detection system (ECL Plus; Amersham Biosciences, Piscataway, NJ) and exposed to single-emulsion film (Biomax MR; Sigma). Band intensities were determined using software developed by Wayne Rasband (ImageJ; National Institutes of Health, Bethesda, MD; available at http://rsb.info.nih.gov/ij/index.html).
Animals
Female C57BL/6 mice, 6–8 weeks of age, were used in this study. All mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and were maintained in the University of Florida Health Science Center in the animal care facilities. They were handled in accordance with the ARVO statement for Use of Animals in Ophthalmic and Vision Research and the guidelines of the Institutional Animal Care and Use Committee at the University of Florida.
PS1 Overexpression Studies
For detection of PS1 overexpression two groups of nonlasered mouse eyes received AAV2-PS1 or AAV2 (n = 6 in each group). Subretinal injection was achieved as previously described.32 In brief, an aperture within the dilated pupil was made through the cornea with a 30½-gauge disposable needle and a 33-gauge unbeveled blunt-tip needle on a syringe (Hamilton; Fisher Scientific, Orlando, FL) was introduced through the corneal opening into the subretinal space. Each eye received 1 μL of vector at 4.36 × 1012 genome copies per mL (this titer was deemed optimal from exploratory studies). Animals were euthanized after 5 weeks for immunohistochemistry and qRT-PCR.
Laser-Induced CNV Mouse Model
The laser procedure was undertaken as previously described.33,34 Briefly, mice were anesthetized with a mixture of ketamine (80 mg/kg) and xylazine (10 mg/kg) and their pupils dilated with tropicamide (0.5%) and phenylephrine (2.5%). Under the fundus microscope an argon green ophthalmic laser, coupled to a slit lamp set to deliver a 50-ms pulse at 200 mW with a 50 μm spot size, was used to rupture Bruch's membrane in three quadrants of the right eye located approximately 50 mm from the optic disc at relative positions of 9, 12, and 3 o'clock. The left eye served as an untreated control. Animals were divided into 7 groups of 12 animals that received either saline (subretinal or intravitreal as a control), PEDF, the γ-secretase inhibitor DAPT, PEDF plus DAPT, AAV2, and AAV2-PS1. AAV2-PS1, AAV2 without PS1 gene, or saline were delivered by subretinal injection at 3 weeks before induction of CNV as described above. PEDF (80 ng/μL), DAPT (16 ng/μL), or PEDF + DAPT were given as a single 1-μL intravitreal injection immediately after laser injury. For PS1 and nicastrin knockdown studies 1 μL of predesigned siRNAs plasmids (Silence Select, Invitrogen) against mouse PS1 (s72263; Applied Biosystems), nicastrin (s81924; Applied Biosystems), or plasmids (Silence Select) negative control siRNA (4404020; Applied Biosystems) at a concentration of 2 μg/μL in transfection agent (siPORT NeoFX; Applied Biosystems) was given by intravitreal injection at the time of laser injury.
Mice were euthanized 14 days after laser injury. The eyes were enucleated and the RPE/choroid/sclera prepared. For measuring lesion volume we used a vascular specific dye (Ricinus Communis Agglutinin I; Vector Laboratories, Inc.) conjugated to rhodamine, to label whole flat mounts of RPE/choroid/sclera which were incubated for 30 minutes at room temperature in 1:400 of 10 mM HEPES, 150 mM NaCl and 0.1% Tween 20. The tissues were covered in aqueous mounting medium (VectaShield; Vector Laboratories, Inc.) for observation on a confocal microscope (Olympus DSU-Olympus IX81; Olympus America, Inc., Center Valley, PA). Digital images were captured by using imaging software (SlideBook 4.2; Intelligent Imaging Innovations, Inc., Denver, CO) in a three-dimensional stacked manner to facilitate volumetric analysis from experimental and control samples with identical photomultiplier tube gain settings. The confocal images were then processed identically in experimental and control eyes and measured using software developed by Wayne Rasband (ImageJ; National Institutes of Health, Bethesda, MD; available at http://rsb.info.nih.gov/ij/index.html).
Immunohistochemistry
Mice were euthanized by anesthetizing with isoflurane followed by cervical dislocation. The eyes were enucleated and fixed in 4% paraformaldehyde/PBS overnight. The anterior segment, lens, and vitreous were removed and the posterior eye cups were prepared for cryostat sections, paraffin sections, or retinal flat mounts The sections or flat mounts were washed with PBS then permeabilized with 0.2% Triton X-100 and nonspecific binding blocked by 10% normal goat serum in PBS. Samples then received primary antibody for 16 hours at 4°C. The primary antibodies were polyclonal anti-PS1 (1:2000), rat anti-HA (1:500), rabbit anti-VEGF (1:300), rabbit anti-angiogenin 1 (1:500), anti-NFκB (1:500), mouse anti-F4/80 (1:500), or rabbit anti-Iba1 (1:200). After primary incubation, tissues were washed and incubated for 1.5 hours at room temperature with the appropriate secondary antibody at 4°C with 0.2% Triton X-100. The secondary antibody was Cy3 conjugated goat anti-rabbit or mouse IgG (1:250). After washing, specimens were mounted in aqueous mounting medium (VectaShield; Vector Laboratories, Inc.) and coverslipped for observation by confocal microscopy. All confocal images were acquired with identical exposure settings.
qRT-PCR Analysis In Vivo and In Vitro
Total mRNA was prepared from both mouse neural retinas and RPE/choroid, and cultured cells using a reagent (Trizol; Invitrogen). Total RNA was extracted using isopropanol precipitation. Pellet was resuspended in 0.1% DEPC water and stored at −80°C. mRNA from each sample was reverse transcribed into cDNA using a cDNA synthesis kit (iScript cDNA Synthesis Kit, 170-8890; Bio-Rad) following manufacturer's instructions. Quantitative PCR was performed using a PCR mix (SsoFast EvaGreen Supermix, 172-5201; Bio-Rad) with designed primers. All primers, mouse presinilin 1 (mPS1), superoxide dismutanase 2 (SOD2), monocyte chemoattractant protein 1 (MCP-1), CXCL8/interleukin-8 (IL-8), NFκB, and an internal control of GAPDH were designed using NCBI and produced by Sigma-Aldrich. The primers are as follows: mouse presenilin 1 (forward: 5′-AGGCGAAGAGTCGTATGGCGC-3′, reverse: 5′-TGTTCGCGGCAGTGCCACAT-3′); mouse superoxide dismutanase 2 (forward: 5′-ACGACTATGGCGCGCTGGAG-3′, reverse: 5′-CTTGGCCAGAGCCTCGTGGT-3′), mouse monocyte chemoattractant protein 1 (forward: 5′-AGTTGCCGGCTGGAGCATCC-3′, reverse: 5′-TCTTTGGGACACCTGCTGCTGG-3′); bovine CXCL8/interleukin-8 (forward: 5′-AGCTGGCTGTTGCTCTCTTGGC-3′; reverse: 5′-GGGGTGGAAAGGTGTGGAATGTG-3′), NFκB (mouse, forward: 5′-TGTCTGCTGCTGCTGGTGGC-3′, reverse: 5′-AAGCAGGCAGCCAGCCAAGG-3′; bovine, forward: 5′-CCAGAATGGCAGAAG ACGACCCG-3′, reverse: 5′-GGGGCCTTCACACACGTAACGG-3′); GAPDH internal control (bovine, forward: 5′-AGGTGGTGAAGCAGGCGTCA-3′, reverse: 5′-CACCACCCTGTTGCTGTAGCCA-3′; mouse, forward: 5′-CCCAGCAAGGA CACTGAGCAAGAG-3′, reverse: 5′-CTAGGCCCCTCCTGTTATTATGGGG-3′). The reactions were run in a PCR system (7500 Fast Real Time PCR System; Applied Biosciences) for 10 minutes at 95°C followed by 40 cycles of amplification for 15 seconds at 95°C and 1 minute at 60°C. The raw data and comparative CT analysis (ΔΔCT) was performed using software (7500 v2.0.1; Intelligent Imaging Innovations, Inc.). The housekeeping gene, GAPDH, was used as the reference gene for the analysis. All reactions were performed in triplicate. The analysis indicated fold difference in gene expression.
Assay for In Vivo Superoxide Generation
The in situ production of superoxide radicals was evaluated using the superoxide indicator, hydroethidine as previously described.35,36 Superoxide oxidizes hydroethidine to yield a red fluorescent signal at approximately 600 nm. Animals were treated with saline, PEDF, DAPT, PEDF + DAPT, AAV2, and AAV2-PS1 as described above. Each group consisted of six mice. Mice received two intraperitoneal injections, 15 minutes apart, of freshly prepared hydroethidine (20 mg/kg; Invitrogen) and were euthanized 18 hours after injection. For quantification of the level of ethidium, posterior eye cups were rinsed and homogenized with a polytron homogenizer in 1 mL of buffer (50 mM ammonium acetate and 150 mM NaCl, pH 7.4), and fluorescence intensity was measured on a fluorescent plate reader (BioTek, Winooski, VT) using a spectrofluorometer (FLUOstar Optima; BMG Labtechnologies, Cary, NC). The relative fluorescence intensity was calculated by normalizing to protein concentration. For microscopic observations eyes were rapidly removed and fixed in 4% paraformaldehyde for 20 minutes at 22°C, rinsed with PBS, and retinal flat mounts were prepared. After rinsing in PBS, tissues were examined by fluorescence microscopy (excitation: 543 nm, emission: 610 nm) and all images were acquired in the frame scan mode with the same exposure time.
siRNA Treatment
Microvascular endothelial cells were transfected with duplex oligoribonucleotides against PS1 (Stealth Select RNAi, s224427; Invitrogen), NCT (s23706; Invitrogen), Aph-1 (s27450; Invitrogen), Pen-2 (s31661; Invitrogen), or with scrambled siRNA (12,935-300, Invitrogen) for 48 hours, after a further 24-hour incubation in endothelial cell basal medium with growth supplement (Invitrogen). Cells were then treated with growth factors as described above.
Assay for γ-Secretase Activity
γ-Secretase activity was determined using a fluorometric γ-secretase activity kit according to the manufacturer's instructions (R&D Systems) as we have previously described.10 The level of secretase enzymatic activity in the cell lysate is proportional to the fluorometric reaction.
Statistical Analysis
All experiments were repeated at least three times and were assessed using a Student's t-test plus ANOVA for multiple comparisons. Results are expressed as mean ± SEM. The Mann-Whitney test was used to determine statistical significance of the laser densitometry data. Statistical analysis was performed using statistical software (Prism 5; GraphPad Software, La Jolla, CA) with differences of P < 0.05 considered statistically significant.
Results
AAV-Mediated Overexpression of PS1 in Vascular Cells
To achieve vascular endothelial transfection, wild type AAV2 was modified with tyrosine to phenylalanine capsid mutations (AAV2 quadYF) to enhance infection efficiency as endothelial cells are typically refractive to viral gene transfer. The VE-cadherin promoter was selected to ensure endothelial cell specific expression of PS1 (Fig. 1A). Viral infection of retinal endothelial cells with AAV2 quadYF-PS1 resulted in a dose-dependent increase in PS1 expression which was greatest 48 hours post infection using a MOI of 10,000 and was increased by > 100% when compared with AAV2 quadYF without PS or untreated control (Fig. 1B). Control AAV2 infection did not significantly increase PS1 expression compared with control. Immunohistochemical staining of the HA tag demonstrated significant infection of > 85% of cells (Fig. 1C).
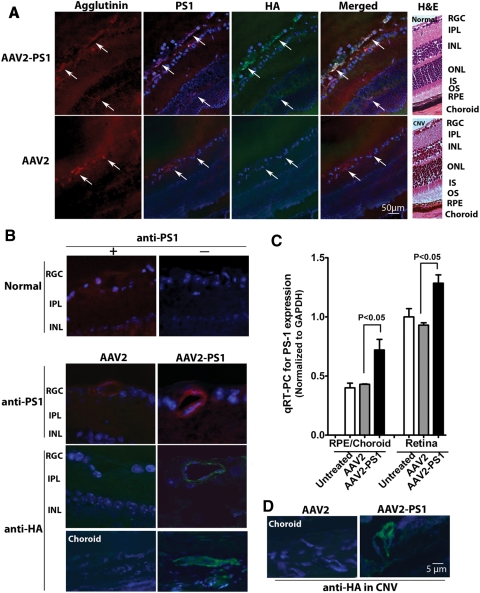
Subretinal injection of AAV2 quadYF-PS1 resulted in elevated expression of PS1 specifically within both the retinal and choroidal vasculature (Fig. 2). Normal retina expressed a low level of endogenous PS1 which was similar to that observed in eyes receiving subretinal injection of control AAV2 (Fig. 2A). This was confirmed by immunostaining for the HA tag which demonstrated robust expression of HA specifically in both retinal and choroidal capillaries which was absent in normal eyes (data not shown) and eyes receiving control AAV2. Increased expression of PS1 in eyes receiving AAV2 quadYF-PS1 was confirmed by qRT-PCR which revealed increased PS1 mRNA expression of 67% and 37% in the RPE/choroid and neural retina, respectively, compared with control (Fig. 2B). Because the tissue preparations are dominated by nonvascular tissue, expression levels in vascular endothelial cells would be expected to be much greater but similar to that observed in our cell culture studies (Fig. 1). Because the focus of this study is on the role of PS1 in CNV we were also able to demonstrate high levels of the HA tag in vessels associated with CNV lesions (Fig. 2C).
Figure 2.
Expression of PS1 in the retinal and choroidal vasculature after subretinal delivery of AAV2 quadYF-PS1. Subretinal injections of AAV2 quadYF-PS1, control AAV2, or saline vehicle were performed in mouse eyes and the animals euthanized 5 weeks later. (A) Representative low-power fluorescence photomicrographs of transverse sections showing immunostaining for PS1 (red) and HA (green) in the retinal and choroidal vessels. Representative hematoxylin and eosin (H&E) sections are shown on the right to provide orientation. (B) Higher power of images shown in (A). (C) Expression of PS1 in neural retina and RPE/choroid preparations normalized against GAPDH. PS1 mRNA expression increased 67% and 37% in the RPE/choroid and neural retina respectively compared with control mRNA levels (mean ± SEM, n = 6). (D) Representative immunostaining for HA in CNV lesions in which laser burns were applied to the retina 3 weeks after the vector injection.
γ-Secretase and PS1 Suppress In Vitro and In Vivo Angiogenesis
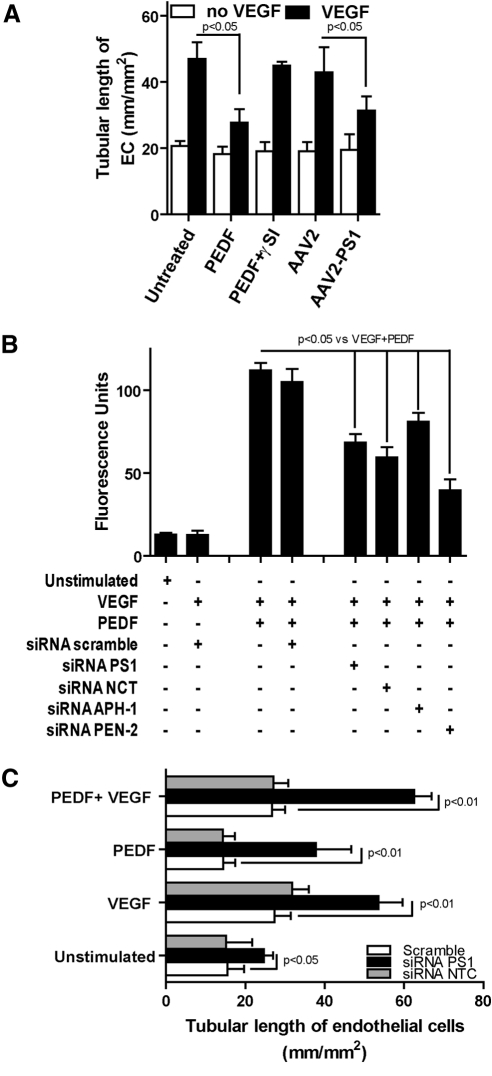
As we have previously reported,10 PEDF inhibited VEGF-induced tubule formation and this was blocked by a γ-secretase inhibitor (Fig. 3A). Overexpression of PS1 using AAV2 quadYF caused a greater than 50% reduction in in vitro angiogenesis compared with control (Fig. 3A). To better define the role of PS1 in the inhibition of angiogenesis, we used siRNA to knockdown each component of the γ-secretase complex. Previously, we showed that these siRNAs knockdown greater than 70% of their target17 and that PEDF alone increases γ-secretase activity through the translocation of the constituent proteins to the plasma membrane.10 Knockdown using each of the siRNA components of γ-secretase significantly reduced the PEDF-induced activity of the γ-secretase complex by approximately 50% compared with control (Fig. 3B). Furthermore, knockdown of PS1, but not nicastrin, blocked PEDF-induced inhibition of tube formation in the in vitro angiogenesis assay and furthermore increased tube formation in untreated cells in the absence of exogenous growth factors (Fig. 3C). This identifies PS1 as the major effector in γ-secretase regulation of angiogenesis.
Figure 3.
γ-Secretase and PS1 suppress in vitro angiogenesis. (A) Endothelial cells were either pre-exposed to PEDF, DAPT, or PEDF + DAPT in the presence or absence of VEGF for 24 hours, or infected with control AAV2 or AAV2 quadYF-PS1 and exposed to VEGF 24 hours later. After overnight incubation with growth factors, cells were transferred to basement membrane matrix (Matrigel; Collaborative Research) and tubule length (mm/mm2) was measured 48 hours later. Increased γ-secretase activity and overexpression of PS1 inhibited VEGF-induced in vitro angiogenesis (mean ± SEM, n = 4). (B) Endothelial cells were transfected with RNAi duplex oligoribonucleotides against PS1, nicastrin, Aph-1, Pen-2, or scrambled siRNA. Cells were exposed to PEDF in the presence or absence of VEGF and γ-secretase activity determined by ELISA (mean ± SEM, n = 4). (C) Endothelial cells were transfected with PS1, nicastrin, or scrambled siRNA (12935-300, Invitrogen) for 48 hours followed by overnight exposure to VEGF, PEDF, VEGF + PEDF, or saline control before the basement membrane matrix (Matrigel) in vitro angiogenesis assay. PS1 knockdown blocked the inhibitory effect of PEDF on VEGF-induced angiogenesis (mean ± SEM, n = 4).
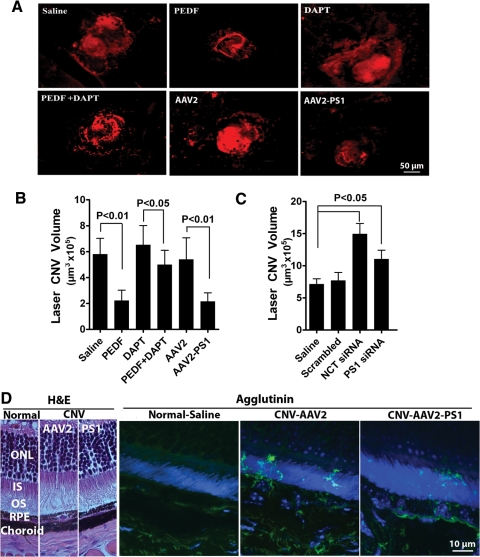
We next assessed the γ-secretase/PS1 modulation of angiogenesis in the mouse CNV model. Intravitreal injection of PEDF significantly reduced CNV lesion volume by greater than 60% and this was blocked if PEDF was injected together with the γ-secretase inhibitor DAPT (Fig. 4A–C). PS1 overexpression after subretinal infection with AAV2 quadYF-PS1 also reduced lesion volume by greater than 60% compared with saline-treated controls. Because the visualization of vascular ingress into the outer retina may be lost in flat mounts of the posterior cup transverse sections of retina and choroid in which the vasculature had been fluorescently stained were also examined. Control AAV2 quadYF-infected eyes displayed extensive ingrowth of vessels into the photoreceptor layer and that this was greatly reduced in eyes infected with AAV2 quadYF-PS1 (Fig. 4B). To further confirm a role for PS1 in CNV we assessed the effect of siRNA against PS1 and nicastrin on CNV lesion volume. In agreement with our in vitro results (Fig. 2A), both PS1 and nicastrin knockdown significantly increased CNV lesion volume with the effect being much greater with nicastrin knockdown relative to untreated control eyes (Fig. 4D).
Figure 4.
γ-Secretase and PS1 suppress CNV. (A) Representative confocal images of laser-induced CNV lesions in eyes. Eyes had either received intravitreal injection of PEDF, DAPT, or PEDF + DAPT at the time of laser injury, or AAV2 infection 3 weeks before receiving laser burns. Saline alone acted as the control. Animals were euthanized 14 days post laser injury and RPE choroidal flat mounts were stained with a vascular specific marker, agglutinin-TRITC conjugate (red) to visualize the CNV lesions by confocal microscopy. Note the reduction in lesion size in mouse eyes treated with PEDF or AAV2-PS1 and that the protective effect of PEDF was blocked by the addition of γ-secretase inhibitor. (B) Quantitative assessment using confocal microscopy to measure the volume of the vascular lesions described in (A) (mean ± SEM, n = 12). (C) For PS1 and nicastrin knockdown studies 1 μL of siRNAs against mouse PS1 nicastrin or negative control siRNA at a concentration of 2 μg/μL was given by intravitreal injection at the time of laser injury. Quantitative assessment was undertaken using confocal microscopy to measure the volume of the CNV lesions (mean ± SEM, n = 12). (D) Representative micrographs of transverse sections through the retina and choroid stained with the vascular fluorescent dye, agglutinin-FITC conjugate (green) and counter nuclear stained with DAPI which demonstrate a significant suppression of choroidal vascular outgrowth into the photoreceptor layer by overexpression of PS1. Representative H&E sections are shown on the left to provide orientation.
PS1 Overexpression Decreases the Expression of VEGF and Angiogenin 1 in Laser-Induced CNV
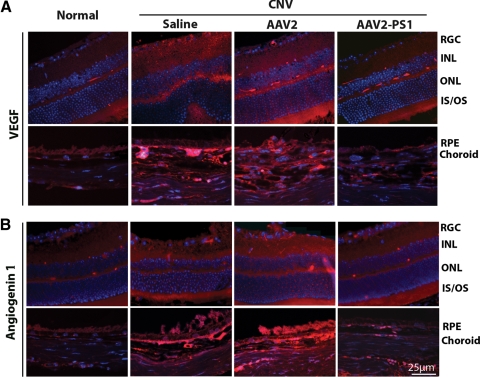
We focused on the expression of two angiogenic factors after PS1 overexpression. It is well recognized the VEGF is an important player in CNV but more recently there is an increasing literature emphasizing the importance of angiogenin 1.37,38 Immunohistochemistry demonstrated very faint staining for VEGF in normal eyes and, as expected, VEGF was increased both in the CNV lesion and in the neural retina in saline and control AAV2 treated eyes (Fig. 5A). The upregulation of VEGF was found predominantly within the inner and outer plexiform layers, photoreceptor layer, RPE, and choroid associated with areas of laser damage. By contrast, eyes receiving AAV2 quadYF-PS1 showed diminished staining for VEGF similar to that seen in normal eyes indicating that overexpression of PS1 reduced VEGF levels both at the site of the laser lesion and within the overlying retina (Fig. 5A). A similar staining pattern was observed for angiogenin 1 which was increased in untreated and control AAV2-treated eyes and decreased in eyes overexpressing PS1 (Fig. 5B).
Figure 5.
PS1 regulates the expression of the proangiogenic factors, VEGF and angiogenin 1, in CNV. Eyes had received subretinal AAV2 infection 3 weeks before receiving laser burns. Saline alone acted as the control. Animals were euthanized 14 days post laser injury, eyes were fixed, and transverse sections were stained (red) for (A) VEGF and (B) angiogenin 1. Sections were counterstained with DAPI to view nuclei (blue). The representative micrographs show that both VEGF and angiogenin 1 levels are decreased in eyes overexpressing PS1.
γ-Secretase and PS1 Suppress Superoxide Anion Generation in Laser-Induced CNV
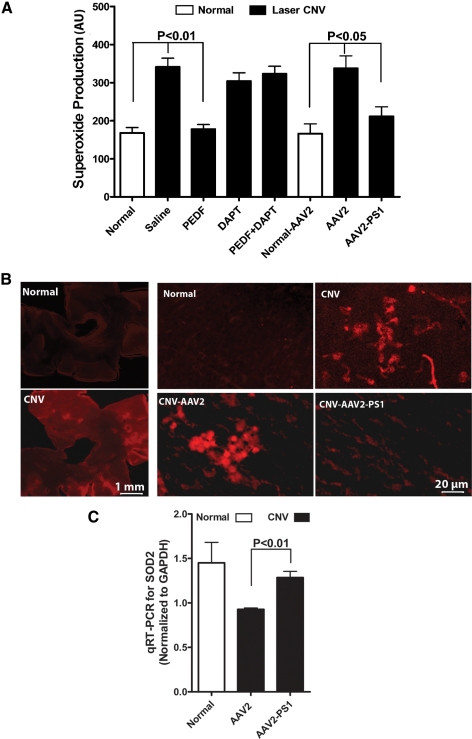
Because PEDF has previously been reported to reduce oxidative stress in retinal cells28,39–41 we next determined if this was via a γ-secretase/PS1-dependent mechanism. Eyes with laser-induced CNV either untreated or receiving saline, γ-secretase inhibitor, or preinfected with control AAV2 showed a greater than 100% increase in superoxide anion levels (Fig. 6A). In marked contrast, this was reduced to baseline levels in eyes receiving intravitreal PEDF or overexpressing PS1. The inhibitory effect of PEDF was blocked by the γ-secretase inhibitor DAPT and superoxide levels were similar to that observed in untreated CNV eyes. These observations were supported by flat mount preparations which demonstrated reduced superoxide reactivity in PS1 overexpressing eyes compared with untreated CNV eyes or eyes receiving AAV2 (Fig. 6B). qRT-PCR demonstrated a reduction in SOD2 expression in control CNV eyes compared with normal eyes and that this decrease was prevented by overexpression of PSI (Fig. 6C).
Figure 6.
PEDF and PS1 overexpression modulate superoxide production and antioxidant enzyme expression. Eyes had either received intravitreal injection of PEDF, DAPT, or PEDF + DAPT at the time of laser, or AAV2 infection 3 weeks before receiving laser burns. Saline alone acted as the control. Animals were euthanized 14 days post laser injury. (A) Quantification of the level of superoxide in the posterior eye cup. Relative fluorescence intensity was calculated by normalizing to protein concentration. PEDF and PS1 overexpression reduced superoxide in CNV eyes. (B) For microscopic observations eyes treated with hydroethidine were fixed in 4% paraformaldehyde and retinal flat mounts were prepared. Tissues were examined by fluorescence microscopy (excitation: 543 nm, emission: 610 nm) and all images were acquired in the frame scan mode with the same exposure time. A low power image of a retinal flat mount from a CNV eye and a DAPI-stained flat mount are shown on the left. (C) qRT-PCR of the posterior cup demonstrated that SOD2 is reduced in CNV eyes and that this can be reversed by overexpression of PS1 (mean ± SEM, n = 12).
PS1 Regulation of Cytokines and Proinflammatory Factors
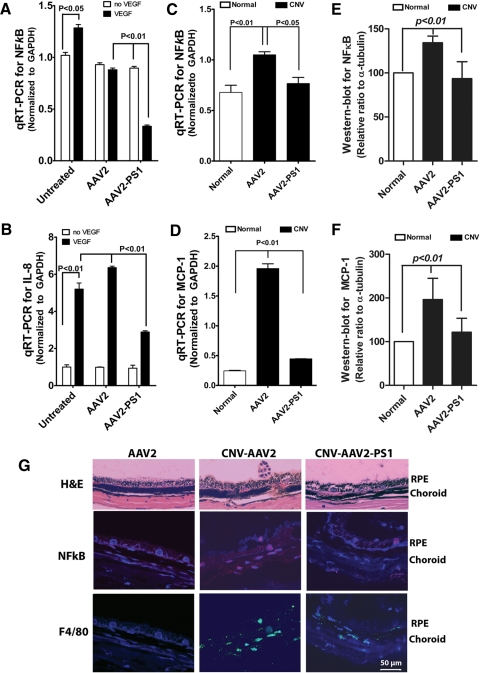
Given the association between proinflammatory factors and ocular angiogenesis29,41,42 we next examined whether PS1 overexpression could reduce proinflammatory markers both in vitro and in vivo. qRT-PCR revealed that overexpression of PS1 significantly reduced VEGF-induced NFκB and IL-8 in cultured endothelial cells (Figs. 7A, 7B). Similarly, overexpression of PS1 reduced NFκB in the laser CNV model and exhibited a reduction in MCP-1 compared with AAV2 control treated eyes (Figs. 7C, 7D). In parallel immunohistochemistry studies, we observed that NFκB immunostaining was accompanied by a large number of F4/80 positive cells in CNV eyes receiving either saline or control AAV2 (Fig. 7E). By contrast, immunoreactivity for both NFκB and F4/80 positive cells was greatly reduced in eyes overexpressing PS1.
Figure 7.
PS1 regulates the expression of cytokines/proinflammatory factors in vitro and in vivo. (A, B) Cultured microvascular endothelial cells were either infected with control AAV2 or AAV2 quadYF-PS1 and after 24 hours were either exposed to VEGF or vehicle control for 48 hours and total mRNA then extracted for qRT-PCR. Overexpression of PS1 significantly reduced VEGF-induced expression of NFκB (A) and IL-8 (B) (mean ± SEM, n = 3). (C, D) qRT-PCR for NFκB (C) and MCP-1 (D) in posterior eye cups of mice that had received subretinal AAV2 infection 3 weeks before receiving laser burns. Saline alone acted as the control. Animals were euthanized 14 days post laser injury. Overexpression of PS1 significantly reduced expression of NFκB and MCP-1 (mean ± SEM, n = 6). (E, F) Western blot analysis from mouse retinas in (C) and (D) showing confirmation that overexpression of PS1 reduced protein expression of NFκB and MCP-1. (G) H&E and immunohistochemical staining for NFκB and the macrophage marker F4/80 in eyes treated as described in (C) showing decreased staining in eyes overexpressing PS1 (mean ± SEM, n = 6).
PS1 Overexpression Suppresses Microglia Activation in Laser-Induced CNV
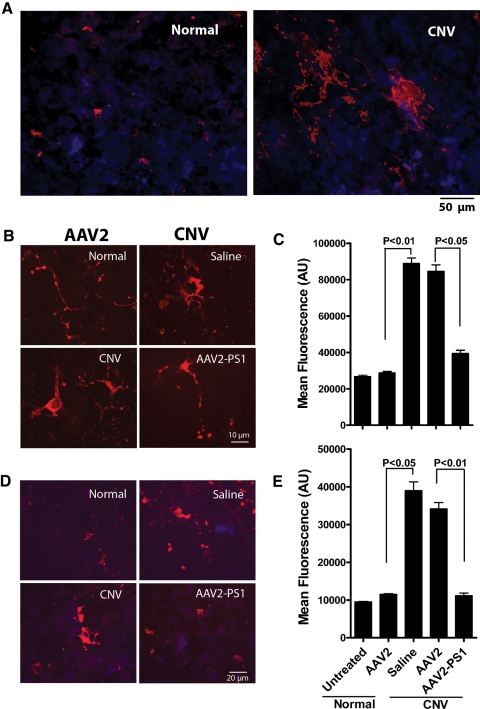
Due to the increased evidence that microglial activation is associated with ocular angiogenesis,43 we explored whether PS1 overexpression can suppress microglial activation. In both neural retina and RPE/choroid flat mounts of untreated or control virus treated CNV eyes, microglia exhibited a much larger cell body exhibiting cell processes typical of activated microglia (Figs. 8A, 8B). Semiquantitative analysis of the flat mounts demonstrated that PS1 overexpression suppressed microglia activation by 1.1-fold in the retina and 2.7-fold in RPE/choroid compared with CNV eyes treated with saline or control AAV2 (P < 0.05) (Figs. 8C, 8D).
Figure 8.
Overexpression of PS1 suppresses activation of microglia in CNV. Eyes had received subretinal AAV2 infection 3 weeks before receiving laser burns. Saline alone acted as the control. Animals were euthanized 14 days post laser injury, eyes were fixed and retina (A) and (B) or RPE/choroid (D) flat mounts were stained for a microglial marker (red) and DAPI (blue). (A) Low power image of (B). The representative micrographs indicate microglial activation in CNV eyes and this is reduced in eyes overexpressing PS1. (C) Quantitative analysis of fluorescence intensity in eight random fields from six eyes per group treated as described above. (D) Representative flat mounts of RPE/choroid showing reduced microglial activation in CNV eyes overexpressing PS1. (E) Semiquantitative analyses of fluorescence intensity in eight random fields from six eyes that confirm the immunostaining in (D).
Discussion
Our results demonstrate that either γ-secretase upregulation by PEDF or PS1 overexpression suppress VEGF-induced angiogenesis in cultured cells and in the laser-induced CNV model. Decreased angiogenesis was associated with a reduction in the expression of angiogenic factors and reduced oxidative stress. Furthermore, PS1 overexpression reduced proinflammatory factors and microglial activation in eyes with CNV. By contrast, angiogenesis was increased by inhibition of either PS1 or nicastrin. Taken together these results indicate that PEDF inhibits CNV via upregulation of γ-secretase activity and that elevated γ-secretase or PS1 offer therapeutic modalities for the treatment of CNV. In addition, results suggest that the use of γ-secretase inhibitors, currently a potential therapeutic option for Alzheimer's disease (AD),44 may actually exacerbate the progression of CNV in these individuals. While clinical trials using semagacestat (a γ-secretase inhibitor) in AD patients did not specifically assess increases in CNV, they were prematurely stopped because of the detrimental cognitive and functional effects of the drug.44
There is considerable evidence that γ-secretase and its component proteins have a potential role in angiogenesis.14 PS1 knockout mice exhibit abnormal blood vessel development45 and Aph1A knockout mice fail to develop an organized vascular system.46 PS1 regulates the growth and differentiation of endothelial progenitor cells.47 The transcription factors Ets and CREB are known to regulate expression of PS and these elements are themselves regulated by a variety of growth factors including VEGF.48,49 Recently we demonstrated that γ-secretase is important for regulating VEGF-induced vascular permeability.50 Furthermore, there is considerable evidence to support a critical role for Notch, which is cleaved by γ-secretase in developmental, adult, and pathologic angiogenesis.51,52 It has also been proposed that γ-secretase regulation of angiogenesis may be VEGFR1-dependent rather than Notch-dependent, however, there is crosstalk between Notch and VEGF receptors and Notch is known to regulate both VEGF and VEGFR expression in a variety of vascular and nonvascular cells.14
The mechanism by which γ-secretase and/or PS1 regulate angiogenesis is unclear especially given the ever increasing number of substrates for γ-secretase.14 Notch activation and γ-secretase are critical for cell-autonomous notch signaling as they regulate endothelial cell branching and proliferation during vascular tubulogenesis.53,54 Furthermore, activation of Notch by Dll4 regulates the formation of tip cells to control vessel sprouting and branching55 and recently, both Notch and Dll4 have been shown to be important regulators of choroidal neovascularization.56,57 An alternative pathway that we have identified is via VEGFR1-mediated inhibition of VEGF-induced angiogenesis.10,17,50 We have shown that PEDF is a potent endogenous anti-angiogenic factor that can inhibit VEGF-induced vascular permeability and angiogenesis due to γ-secretase-dependent regulation of the cleavage and translocation of VEGFR1, because PEDF action is blocked by either pharmacological inhibition of γ-secretase or by siRNA γ-secretase inhibition.14,50 It is possible that other receptors or target proteins may influence angiogenesis because γ-secretase is now reported to have over 50 different substrates.14 Furthermore, it has recently been identified that PS1 can act separately from the γ-secretase complex, possibly as an adaptor molecule facilitating protein trafficking and protein-protein interactions although the underlying mechanisms remain unclear.18–20 Some consensus exists that the mechanism involves the association between full length PS (Fl.PS1). In support of this, we recently reported that Fl.PS1 acts as an adaptor to facilitate the association of vascular endothelial-protein tyrosine phosphatase (VE-PTP) with VEGFR1, thus promoting VEGFR1 dephosphorylation.17 This would help explain how viral upregulation of FL.PS1 in this study was able to inhibit CNV.
It is important to note that γ-secretase activity does not necessarily fully correlate with siRNA knockdown of each its components and that expression of each component of the γ-secretase complex is coordinately regulated and our data would support this. Reciprocal regulation between NCT and PS plays an important role in determining the activity of the γ-secretase complex.58 Downregulation of NCT destabilizes PS and lowers levels of the γ-secretase complex59 while PS1 knockdown is closely associated with downregulation of NCT maturation.58 Thus it is not surprising that knockdown of NCT or PS1 enhance laser-induced CNV. The ability of NCT knockdown to cause a greater increase in CNV than PS1 knockdown likely represents the fact that loss of NCT will reduce both substrate recognition and γ-secretase activity. It was surprising that NCT had no significant effect on in vitro tubule formation. Although highly reproducible we do not have a clear answer for this and can only speculate it reflects a difference between the in vitro model and the in vivo model or species variation. PS1 or nicastrin knockdown have both been shown to result in reduction of Pen-2 levels and there is some evidence that Pen-2 plays a role in PS1 endoproteolysis.60,61 In this study we have targeted Aph-1αL because it has been suggested as the dominant form of Aph-1 but recent studies reports that multiple isoforms (αL, αS, and β) of Aph-1 can replace each other in the γ-secretase complex.62,63
Overexpression of PS1 was specifically targeted to retinal and choroidal vascular endothelial cells. It is well recognized that endothelial cells are refractory to infection by the standard available AAV serotypes and to overcome this it was necessary to test different capsid mutations. We identified an AAV serotype 2 quadruple tyrosine to phenylalanine (Y-F) capsid mutant (Y444F, Y500F, Y700F, and Y704F) as effective for the transfection of endothelial cells. We packaged PS1 with a VE-cadherin promoter to ensure that PS1 expression was restricted to vascular endothelial cells. This novel viral construct offers great potential for the genetic manipulation of both ocular and nonocular vasculature.
Oxidative stress and inflammation represent significant features of CNV lesions and it has previously been demonstrated that PEDF can prevent this.28,29 Our data confirm a role for γ-secretase in this process as inhibition of γ-secretase blocked the protective effect of PEDF. However, the mechanism by which γ-secretase, and PS1 alone, exert this effect is unclear. In this study, the increase of VEGF and angiogenin 1 (factors associated with AMD and implicated in oxidative stress and inflammatory responses)37,38,64 accompanying laser-induced CNV were ameliorated by upregulation of PS1. It is unclear whether γ-secretase is acting indirectly by modulating signaling pathways that regulate oxidative stress and/or inflammation or is acting directly on a critical substrate. Although γ-secretase has been most extensively studied in AD, its role in oxidative stress and inflammation remains a matter for debate. In contrast to our observations in the eye, γ-secretase inhibition has been reported to protect against oxidative stress in AD.65,66 However, there are reports that PS1 is upregulated in response to oxidative stress suggesting that it may play a protective role.67,68 Progressive inflammation is observed in PS knockout mice69 and there is an association between γ-secretase, inflammation, and the Notch pathway.70,71 Furthermore, Notch has been shown to regulate microglial activation.72
In conclusion, it is evident that γ-secretase plays a critical role in facilitating the antiangiogenic effect of PEDF and that PS1 upregulation is sufficient to prevent CNV. However, although this is associated with reduced oxidative stress and a decreased inflammatory response the precise mechanism(s) involved will require further elucidation.
Footnotes
Supported by NIH Grants EY018358 and EY018358-04S1 (MEB), EY012601 and DK090730 (MBG), EY11123 and EY021721 (WWH), EY021721, AHAF Grant M2009024, and a grant from Research to Prevent Blindness, Inc.
Disclosure: X. Qi, None; J. Cai, None; Q. Ruan, None; L. Liu, None; S.L. Boye, None; Z. Chen, None; W.W. Hauswirth, AGTC Inc. (I); R.C. Ryals, None; L. Shaw, None; S. Caballero, None; M.B. Grant, None; M.E. Boulton, None
References
- 1. de Jong PT. Age-related macular degeneration. N Engl J Med. 2006;355:1474–1485 [DOI] [PubMed] [Google Scholar]
- 2. Zarbin MA. Current concepts in the pathogenesis of age-related macular degeneration. Arch Ophthalmol. 2004;122:598–614 [DOI] [PubMed] [Google Scholar]
- 3. Campa C, Harding SP. Anti-VEGF compounds in the treatment of neovascular age related macular degeneration. Curr Drug Targets. 2011;12:173–181 [DOI] [PubMed] [Google Scholar]
- 4. Penn JS, Madan A, Caldwell RB, Bartoli M, Caldwell RW, Hartnett ME. Vascular endothelial growth factor in eye disease. Prog Retin Eye Res. 2008;27:331–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Afzal A, Shaw LC, Ljubimov AV, Boulton ME, Segal MS, Grant MB. Retinal and choroidal microangiopathies: therapeutic opportunities. Microvasc Res. 2007;74:131–144 [DOI] [PubMed] [Google Scholar]
- 6. Tombran-Tink J. PEDF in angiogenic eye diseases. Curr Mol Med. 2010;10:267–278 [DOI] [PubMed] [Google Scholar]
- 7. Bhutto IA, McLeod DS, Hasegawa T, et al. Pigment epithelium-derived factor (PEDF) and vascular endothelial growth factor (VEGF) in aged human choroid and eyes with age-related macular degeneration. Exp Eye Res. 2006;82:99–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Smith CP, Steinle JJ. Changes in growth factor expression in normal aging of the rat retina. Exp Eye Res. 2007;85:817–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tombran-Tink J, Shivaram SM, Chader GJ, Johnson LV, Bok D. Expression, secretion, and age-related downregulation of pigment epithelium-derived factor, a serpin with neurotrophic activity. J Neurosci. 1995;15:4992–5003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cai J, Jiang WG, Grant MB, Boulton M. Pigment epithelium-derived factor inhibits angiogenesis via regulated intracellular proteolysis of vascular endothelial growth factor receptor 1. J Biol Chem. 2006;281:3604–3613 [DOI] [PubMed] [Google Scholar]
- 11. Ablonczy Z, Prakasam A, Fant J, Fauq A, Crosson C, Sambamurti K. Pigment epithelium-derived factor maintains retinal pigment epithelium function by inhibiting vascular endothelial growth factor-R2 signaling through gamma-secretase. J Biol Chem. 2009;284:30177–30186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wolfe MS. The gamma-secretase complex: membrane-embedded proteolytic ensemble. Biochemistry. 2006;45:7931–7939 [DOI] [PubMed] [Google Scholar]
- 13. Brunkan AL, Goate AM. Presenilin function and gamma-secretase activity. J Neurochem. 2005;93:769–792 [DOI] [PubMed] [Google Scholar]
- 14. Boulton ME, Cai J, Grant MB. gamma-Secretase: a multifaceted regulator of angiogenesis. J Cell Mol Med. 2008;12:781–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. De Strooper B. Nicastrin: gatekeeper of the gamma-secretase complex. Cell. 2005;122:318–320 [DOI] [PubMed] [Google Scholar]
- 16. Taniguchi Y, Kim SH, Sisodia SS. Presenilin-dependent “gamma-secretase” processing of deleted in colorectal cancer (DCC). J Biol Chem. 2003;278:30425–30428 [DOI] [PubMed] [Google Scholar]
- 17. Cai J. Gamma-secretase and presenilin mediate cleavage and phosphorylation of vascular endothelial growth factor receptor-1. J Biol Chem. 2011;286:42514–42523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Scheper W, Zwart R, Baas F. Rab6 membrane association is dependent of Presenilin 1 and cellular phosphorylation events. Brain Res Mol Brain Res. 2004;122:17–23 [DOI] [PubMed] [Google Scholar]
- 19. Kametani F, Usami M, Tanaka K, Kume H, Mori H. Mutant presenilin (A260V) affects Rab8 in PC12D cell. Neurochem Int. 2004;44:313–320 [DOI] [PubMed] [Google Scholar]
- 20. Dumanchin C, Czech C, Campion D, et al. Presenilins interact with Rab11, a small GTPase involved in the regulation of vesicular transport. Hum Mol Genet. 1999;8:1263–1269 [DOI] [PubMed] [Google Scholar]
- 21. Beatty S, Koh H, Phil M, Henson D, Boulton M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2000;45:115–134 [DOI] [PubMed] [Google Scholar]
- 22. Hara R, Inomata Y, Kawaji T, et al. Suppression of choroidal neovascularization by N-acetyl-cysteine in mice. Curr Eye Res. 2010;35:1012–1020 [DOI] [PubMed] [Google Scholar]
- 23. Li Q, Dinculescu A, Shan Z, et al. Downregulation of p22phox in retinal pigment epithelial cells inhibits choroidal neovascularization in mice. Mol Ther. 2008;16:1688–1694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Augustin AJ, Kirchhof J. Inflammation and the pathogenesis of age-related macular degeneration. Expert Opin Ther Targets. 2009;13:641–651 [DOI] [PubMed] [Google Scholar]
- 25. Benny O, Nakai K, Yoshimura T, et al. Broad spectrum antiangiogenic treatment for ocular neovascular diseases. PLoS One. 2010;5:e12515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jin J, Zhou KK, Park K, et al. Anti-inflammatory and antiangiogenic effects of nanoparticle-mediated delivery of a natural angiogenic inhibitor. Invest Ophthalmol Vis Sci. 2011;52:6230–6237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nagineni C, Kommineni V, William A, Detrick B, Hooks J. Regulation of VEGF expressionin human retinal cells by cytokines: implications for the role of inflammation in age-related macular degeneration. J Cell Physiol. 2012;227:116–126 [DOI] [PubMed] [Google Scholar]
- 28. Banumathi E, Sheikpranbabu S, Haribalaganesh R, Gurunathan S. PEDF prevents reactive oxygen species generation and retinal endothelial cell damage at high glucose levels. Exp Eye Res. 2010;90:89–96 [DOI] [PubMed] [Google Scholar]
- 29. Park K, Jin J, Hu Y, Zhou K, Ma JX. Overexpression of pigment epithelium-derived factor inhibits retinal inflammation and neovascularization. Am J Pathol. 2011;178:688–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hauswirth WW, Lewin AS, Zolotukhin S, Muzyczka N. Production and purification of recombinant adeno-associated virus. Methods Enzymol. 2000;316:743–761 [DOI] [PubMed] [Google Scholar]
- 31. Cai J, Ahmad S, Jiang WG, et al. Activation of vascular endothelial growth factor receptor-1 sustains angiogenesis and Bcl-2 expression via the phosphatidylinositol 3-kinase pathway in endothelial cells. Diabetes. 2003;52:2959–2968 [DOI] [PubMed] [Google Scholar]
- 32. Alexander JJ, Umino Y, Everhart D, et al. Restoration of cone vision in a mouse model of achromatopsia. Nat Med. 2007;13:685–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Caballero S, Swaney J, Moreno K, et al. Anti-sphingosine-1-phosphate monoclonal antibodies inhibit angiogenesis and sub-retinal fibrosis in a murine model of laser-induced choroidal neovascularization. Exp Eye Res. 2009;88:367–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shaw LC, Pan H, Afzal A, et al. Proliferating endothelial cell-specific expression of IGF-I receptor ribozyme inhibits retinal neovascularization. Gene Ther. 2006;13:752–760 [DOI] [PubMed] [Google Scholar]
- 35. Komeima K, Usui S, Shen J, Rogers BS, Campochiaro PA. Blockade of neuronal nitric oxide synthase reduces cone cell death in a model of retinitis pigmentosa. Free Radic Biol Med. 2008;45:905–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Usui S, Oveson BC, Lee SY, et al. NADPH oxidase plays a central role in cone cell death in retinitis pigmentosa. J Neurochem. 2009;110:1028–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Oikonomou KA, Kapsoritakis AN, Kapsoritaki AI, et al. Angiogenin, angiopoietin-1, angiopoietin-2, and endostatin serum levels in inflammatory bowel disease. Inflamm Bowel Dis. 2011;17:963–970 [DOI] [PubMed] [Google Scholar]
- 38. Skeie JM, Zeng S, Faidley EA, Mullins RF. Angiogenin in age-related macular degeneration. Mol Vis. 2011;17:576–582 [PMC free article] [PubMed] [Google Scholar]
- 39. Nakamura K, Yamagishi S, Matsui T, et al. Pigment epithelium-derived factor inhibits neointimal hyperplasia after vascular injury by blocking NADPH oxidase-mediated reactive oxygen species generation. Am J Pathol. 2007;170:2159–2170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yamagishi S, Inagaki Y, Nakamura K, et al. Pigment epithelium-derived factor inhibits TNF-alpha-induced interleukin-6 expression in endothelial cells by suppressing NADPH oxidase-mediated reactive oxygen species generation. J Mol Cell Cardiol. 2004;37:497–506 [DOI] [PubMed] [Google Scholar]
- 41. Zhang SX, Wang JJ, Dashti A, et al. Pigment epithelium-derived factor mitigates inflammation and oxidative stress in retinal pericytes exposed to oxidized low-density lipoprotein. J Mol Endocrinol. 2008;41:135–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang JJ, Zhang SX, Mott R, et al. Anti-inflammatory effects of pigment epithelium-derived factor in diabetic nephropathy. Am J Physiol Renal Physiol. 2008;294:F1166–F1173 [DOI] [PubMed] [Google Scholar]
- 43. Unoki N, Murakami T, Nishijima K, Ogino K, van Rooijen N, Yoshimura N. SDF-1/CXCR4 contributes to the activation of tip cells and microglia in retinal angiogenesis. Invest Ophthalmol Vis Sci. 2010;51:3362–3371 [DOI] [PubMed] [Google Scholar]
- 44. Imbimbo BP, Panza F, Frisardi V, et al. Therapeutic intervention for Alzheimer's disease with gamma-secretase inhibitors: still a viable option? Expert Opin Invest Drugs. 2011;20:325–341 [DOI] [PubMed] [Google Scholar]
- 45. Nakajima M, Yuasa S, Ueno M, Takakura N, Koseki H, Shirasawa T. Abnormal blood vessel development in mice lacking presenilin-1. Mech Dev. 2003;120:657–667 [DOI] [PubMed] [Google Scholar]
- 46. Serneels L, Dejaegere T, Craessaerts K, et al. Differential contribution of the three Aph1 genes to gamma-secretase activity in vivo. Proc Natl Acad Sci U S A. 2005;102:1719–1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nakajima M, Ogawa M, Shimoda Y, et al. Presenilin-1 controls the growth and differentiation of endothelial progenitor cells through its beta-catenin-binding region. Cell Biol Int. 2006;30:239–243 [DOI] [PubMed] [Google Scholar]
- 48. Mayo LD, Kessler KM, Pincheira R, Warren RS, Donner DB. Vascular endothelial cell growth factor activates CRE-binding protein by signaling through the KDR receptor tyrosine kinase. J Biol Chem. 2001;276:25184–25189 [DOI] [PubMed] [Google Scholar]
- 49. Murakami Y, Yamagoe S, Noguchi K, et al. Ets-1-dependent expression of vascular endothelial growth factor receptors is activated by latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus through interaction with Daxx. J Biol Chem. 2006;281:28113–28121 [DOI] [PubMed] [Google Scholar]
- 50. Cai J, Wu L, Qi X, et al. PEDF regulates vascular permeability by a gamma-secretase-mediated pathway. PLoS One. 2011;6:e21164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shi W, Harris AL. Notch signaling in breast cancer and tumor angiogenesis: cross-talk and therapeutic potentials. J Mammary Gland Biol Neoplasia. 2006;11:41–52 [DOI] [PubMed] [Google Scholar]
- 52. Gridley T. Notch signaling in the vasculature. Curr Top Dev Biol. 2010;92:277–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sainson RC, Aoto J, Nakatsu MN, et al. Cell-autonomous Notch signaling regulates endothelial cell branching and proliferation during vascular tubulogenesis. FASEB J. 2005;19:1027–1029 [DOI] [PubMed] [Google Scholar]
- 54. Kalen M, Heikura T, Karvinen H, et al. Gamma-secretase inhibitor treatment promotes VEGF-A-driven blood vessel growth and vascular leakage but disrupts neovascular perfusion. PLoS One. 2011;6:e18709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hellstrom M, Phng LK, Hofmann JJ, et al. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445:776–780 [DOI] [PubMed] [Google Scholar]
- 56. Ahmad I, Balasubramanian S, Del Debbio CB, et al. Regulation of ocular angiogenesis by Notch signaling: implications in neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52:2868–2878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Dong X, Wang YS, Dou GR, et al. Influence of Dll4 via HIF-1alpha-VEGF signaling on the angiogenesis of choroidal neovascularization under hypoxic conditions. PLoS One. 2011;6:e18481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Edbauer D, Winkler E, Haass C, Steiner H. Presenilin and nicastrin regulate each other and determine amyloid beta-peptide production via complex formation. Proc Natl Acad Sci U S A. 2002;99:8666–8671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhou S, Zhou H, Walian PJ, Jap BK. CD147 is a regulatory subunit of the gamma-secretase complex in Alzheimer's disease amyloid beta-peptide production. Proc Natl Acad Sci U S A. 2005;102:7499–7504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Steiner H, Winkler E, Edbauer D, et al. PEN-2 is an integral component of the gamma-secretase complex required for coordinated expression of presenilin and nicastrin. J Biol Chem. 2002;277:39062–39065 [DOI] [PubMed] [Google Scholar]
- 61. Takasugi N, Tomita T, Hayashi I, et al. The role of presenilin cofactors in the gamma-secretase complex. Nature. 2003;422:438–441 [DOI] [PubMed] [Google Scholar]
- 62. Chen AC, Guo LY, Ostaszewski BL, Selkoe DJ, LaVoie MJ. Aph-1 associates directly with full-length and C-terminal fragments of gamma-secretase substrates. J Biol Chem. 2010;285:11378–11391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Shiraishi H, Marutani T, Wang HQ, et al. Reconstitution of gamma-secretase by truncated presenilin (PS) fragments revealed that PS C-terminal transmembrane domain is critical for formation of gamma-secretase complex. Genes Cells. 2006;11:83–93 [DOI] [PubMed] [Google Scholar]
- 64. Angelo LS, Kurzrock R. Vascular endothelial growth factor and its relationship to inflammatory mediators. Clin Cancer Res. 2007;13:2825–2830 [DOI] [PubMed] [Google Scholar]
- 65. Jo DG, Arumugam TV, Woo HN, et al. Evidence that gamma-secretase mediates oxidative stress-induced beta-secretase expression in Alzheimer's disease. Neurobiol Aging. 2010;31:917–925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sheng B, Gong K, Niu Y, et al. Inhibition of gamma-secretase activity reduces Abeta production, reduces oxidative stress, increases mitochondrial activity and leads to reduced vulnerability to apoptosis: implications for the treatment of Alzheimer's disease. Free Radic Biol Med. 2009;46:1362–1375 [DOI] [PubMed] [Google Scholar]
- 67. Oda A, Tamaoka A, Araki W. Oxidative stress up-regulates presenilin 1 in lipid rafts in neuronal cells. J Neurosci Res. 2010;88:1137–1145 [DOI] [PubMed] [Google Scholar]
- 68. Tamagno E, Guglielmotto M, Aragno M, et al. Oxidative stress activates a positive feedback between the gamma- and beta-secretase cleavages of the beta-amyloid precursor protein. J Neurochem. 2008;104:683–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Saura CA. Presenilin/gamma-secretase and inflammation. Front Aging Neurosci. 2010;2:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Heneka MT, O'Banion MK, Terwel D, Kummer MP. Neuroinflammatory processes in Alzheimer's disease. J Neural Transm. 2010;117:919–947 [DOI] [PubMed] [Google Scholar]
- 71. Sastre M, Walter J, Gentleman SM. Interactions between APP secretases and inflammatory mediators. J Neuroinflammation. 2008;5:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wei Z, Chigurupati S, Arumugam TV, Jo DG, Li H, Chan SL. Notch activation enhances the microglia-mediated inflammatory response associated with focal cerebral ischemia. Stroke. 2011;42:2589–2594 [DOI] [PubMed] [Google Scholar]