Abstract
The TRK-fused gene (TFG in human, Tfg in rat) was originally identified in human papillary thyroid cancer as a chimeric form of the NTRK1 gene. It was since reported that the gene product (TFG) plays a role in regulating phosphotyrosine-specific phosphatase-1 activity. As shown in the accompanying paper, we produced an antibody to rat TFG and used it to localize TFG to selected neurons in specific regions. In the present study, we mapped the TFG-positive neurons in the brainstem, cerebellum, and spinal cord of rats. In the brainstem, neurons intensely positive for TFG were distributed in the raphe nuclei, the gigantocellular reticular nucleus, the reticulotegmental nucleus of the pons, and some cranial nerve nuclei such as the trigeminal nuclei, the vestibulocochlear nuclei, and the dorsal motor nucleus of the vagus. Purkinje cells in the cerebellum and motor neurons in the spinal anterior horn were also positive for TFG. These results provide fundamental data for studying the functions of TFG in the brain.
Keywords: TFG, brain, neuron, oncogene, immunohistochemistry
I. Introduction
The TRK-fused gene (TFG in human, Tfg in rat) was first identified in human papillary thyroid carcinoma as a fusion partner of the NTRK1 gene [5], which encodes a tyrosine kinase receptor for nerve growth factor [9, 10]. TFG was subsequently found as an oncogenic fusion gene in various cancers, including anaplastic large cell lymphoma [6], myxoid chondrosarcoma [7], and atypical myeloproliferative neoplasms [3]. Recently, the gene product, TFG, was implicated in regulating cargo export at the endoplasmic reticulum [23].
Despite the TFG gene being expressed across several cancerous and normal tissues [13, 14, 20], the function of TFG protein remains unclear. In 2005, Roccato et al. [21] reported that TFG protein interacts with and negatively regulates the SH2 domain-containing phosphotyrosine-specific phosphatase-1 (SHP-1), which is expressed in the hematopoietic system [19, 24], epithelial cells [19], and the nervous system [8, 12]. Therefore, TFG protein may play an important role in these tissues by regulating SHP-1. In addition, the ortholog of TFG in C. elegans, tfg-1, suppresses apoptosis and is essential for normal cell size [4].
Recently, we produced an antibody specific to the rat TFG protein [11]. Immunohistochemistry using the antibody localized TFG to some neurons in restricted regions. The distribution suggested that TFG protein could play an important role in specific neuronal functions. To explore this hypothesis, we mapped the distribution patterns of TFG-positive neurons in rat brainstem, cerebellum, and spinal cord using the TFG-specific antibody.
II. Materials and Methods
Animals
Ten male Wistar rats weighing 200–300 g at the start of the experiment were used in this study. The rats were purchased from Clea Japan (Osaka, Japan). All animal experiment was performed following the PHS Policy on Humane Care and Use of Laboratory Animals, the NIH Guide for the Care and Use of Laboratory Animals (NIH publication No. 85-23, revised 1985), and the Animal Welfare Act (7 U.S.C. et seq.). The animal-use protocol was approved by the Institutional Animal Care and Use Committee of Shiga University of Medical Science. All animals were housed under a 12 hr:12 hr light-dark schedule. Food and water were given ad libitum.
Tissue preparations
Ten male Wistar rats were used in this study. Tissue preparation was performed essentially as reported before [1, 25]. In brief, under pentobarbital anesthesia (80 mg/kg), rats were transcardially perfused with 10 mM phosphate-buffered saline (PBS) followed by ice-cold 0.1 M phosphate buffer (PB; pH 7.4) containing 4% formaldehyde (FA). The brain was removed from each rat and the spinal cord was removed from two rats, and they were postfixed for 24 hr in 0.1 M PB containing 4% FA at 4°C. The tissues were then immersed for at least 48 hr in 0.1 M PB containing 15% sucrose and 0.1% sodium azide for cryoprotection. The tissues were cut into 20-µm thick sections using a cryostat. Sections were used in a free-floating state.
Production and characterization of antibody against TFG proteins
The production and characterization of the antibody to TFG was described previously [11]. In brief, the antisera was raised in rabbits using a synthetic peptide corresponding to the common region of TFG protein and its variant as an antigen (SGPPSAPTEDRSGTP: amino acid number 194–208, Accession number BC078947 on GenBank). This peptide was conjugated to bovine serum albumin (BSA) using glutaraldehyde treatment. Antisera were raised in rabbits by immunizing with this antigenic preparation. The best antiserum was then purified by affinity chromatography using the antigenic peptide (SGPPSAPTEDRSGT) bound to CNBr-activated sepharose gel.
The specificity of the antibody was assessed by western blot analysis and immunoabsorption test. Western blotting revealed two bands with molecular weights of approximately 30 kDa and 50 kDa in the brain homogenate, which correspond in molecular size to the conventional and variant forms of TFG, respectively [11].
Immunohistochemistry
Immunohistochemical staining for TFG was performed as previously described [1, 11, 16, 17, 22, 25]. In brief, the sections were kept at 4°C for at least 4 days in 0.1 M PBS containing 0.3% Triton X-100 (PBST; pH 7.4) before staining. Sections were incubated for 20 min with PBST containing 0.3% H2O2 and 0.1% sodium azide at room temperature to eliminate endogenous peroxidase. After washing with PBST, sections were incubated for 30 min with PBST containing 2% BSA to inhibit non-specific protein binding. The sections were incubated in sequence with the rabbit anti-TFG antiserum (1:5,000–20,000) or the purified TFG antibody (0.1 µg/ml) at 4°C overnight, biotinylated anti-rabbit IgG (1:1,000; Vector Laboratories, Burlingame, CA, USA) for 1 hr at room temperature, and the avidin-biotin-peroxidase complex (1:3,000; Vector Laboratories) for 1 hr at room temperature. PBST containing 0.2% BSA was used to dilute the antibodies and PBST was used to wash the sections between each step. A purple color was developed with 0.02% 3,3'-diaminobenzidine, 0.0045% H2O2, and 0.3% nickel ammonium sulfate in 50 mM Tris–HCl buffer (pH 7.6). The free-floating sections were mounted on gelatin-chrome-coated glass slides and air-dried.
For the immunoabsorption test, the purified antibody was preincubated with the TFG peptide (100 µg/mL) for 2 hr at 4°C, and the sections were then stained as described above. All staining was abolished following preincubation of the antibody with the TFG peptide.
Double fluorescence immunohistochemistry
The colocalization of TFG and serotonin-positive neurons in some midbrain sections was examined using double labeling for TFG and serotonin with fluorescence-labeled secondary antibodies. The sections were incubated with a mixture of the rabbit polyclonal antibody (1:5,000) to TFG and mouse monoclonal antibody to serotonin (1:1,000) [22] for 3 days at 4°C. The sections were incubated for 1 hr with a mixture of Alexa 488-conjugated anti-rabbit IgG (1:500; Molecular Probes, Eugene, OR) and Alexa 555-conjugated anti-mouse IgG (1:500; Molecular Probes) at room temperature. Sections were given three rinses with PBST then mounted on silan-coated glass slides and examined using a confocal microscope (Nikon C1si, TE2000-E, Nikon, Tokyo, Japan). PBST containing 0.2% BSA was used to dilute the antibodies and PBST was used to wash the sections between each step.
Cell counting
The number of TFG- and/or serotonin-immunopositive cells was determined in three 20-µm samples of rat midbrain. Confocal microscopy was used to capture images of fluorescently-labeled neurons in the dorsal, paramedian and median raphe nuclei at levels of –8.30, –8.00, and –7.64 mm from the Bregma [18]. The number of cells positive for TFG, serotonin or both in these images was counted by eye.
III. Results
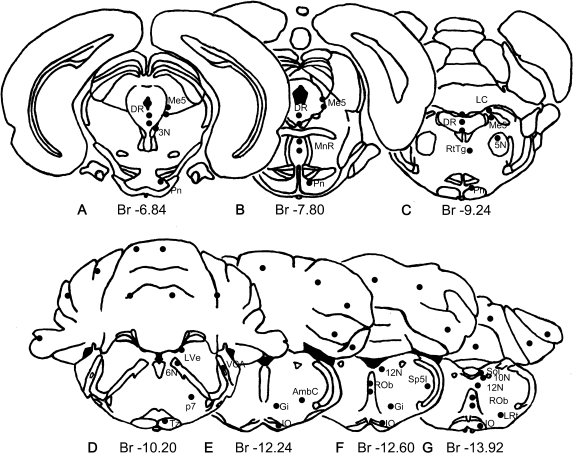
TFG immunoreactivity was detected in neurons of adult rat brain. Figure 1 illustrates the distribution maps of these TFG-positive neurons with seven schematic drawings of coronal sections from the midbrain, pons, medulla and cerebellum, using the atlas of Paxinos and Watson [18] as a reference for the drawings (Fig. 1). The areas with TFG-positive neurons were labeled with dot circles (Fig. 1). Typical examples of TFG-positive neurons are presented in Figures 2 and 3.
Fig. 1.
Mapping of TFG localization in the rat midbrain, pons, medulla and cerebellum. Schematic drawing of rat brain using the Paxinos and Watson Altas as a reference. Br indicates Bregma and the numbers indicate distance (mm) from Bregma.
Abbreviations used in figures: 3N, oculomotor nucleus; 5N, motor trigeminal nucleus; 6N, abducens nucleus; 10N, dorsal motor nucleus of the vagus; 12N, hypoglossal nucleus; AP, area postrema; AmbC, ambiguus nucleus, compact part; CC, central canal; DR, dorsal raphe nucleus; Gi, gigantocellular reticular nucleus; IO, inferior olive; LC, locus coeruleus; LVe, lateral vestibular nucleus; LRt, lateral reticular nucleus; Me5, mesencephalic trigeminal nucleus; p7, perifacial zone; Pn, pontine nuclei; ROb, raphe obscurus nucleus; RtTg, reticulotegmental nucleus of the pons; Sol, nucleus of the solitary tract; Sp5I, spinal trigeminal nucleus, interpolar part; Tz, nucleus of the trapezoid body; VCA, ventral cochlear nucleus, anterior part.
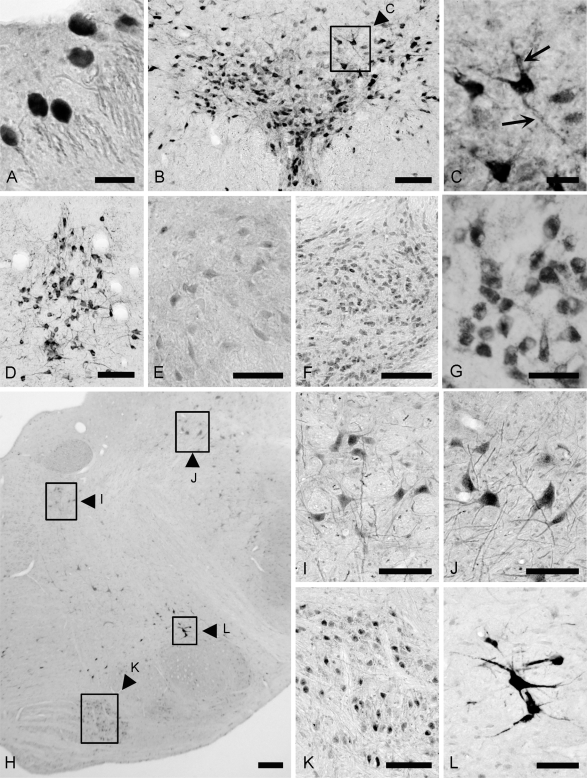
Fig. 2.
Immunohistochemical localization of TFG protein in the nuclei of midbrain (A–F) and pons (G–M) using the TFG-specific antibody. A–F; In the midbrain, intensely stained neurons were seen in the mescenphalic nucleus of the trigeminal nerve (A) and dorsal raphe nucleus (B and C). TFG-immunoreactivity is localized to neuronal somata and proximal processes (arrows in C). Neurons in the median raphe (D) were also intensely stained. TFG-positive neurons were seen in the oculomotor nucleus (E), and the pontine nucleus (F). G–M; In the pons, neurons in the ventral cochlear nucleus (G), the abducens nucleus (H and I), lateral vestibular nucleus (H and J), the nucleus of the trapezoid body (H and K), and the perifacial zone (H and L) were positive for TFG immunoreactivity. Bars=50 µm (A and C); 100 µm (B, D–H, J–M); 200 µm (I).
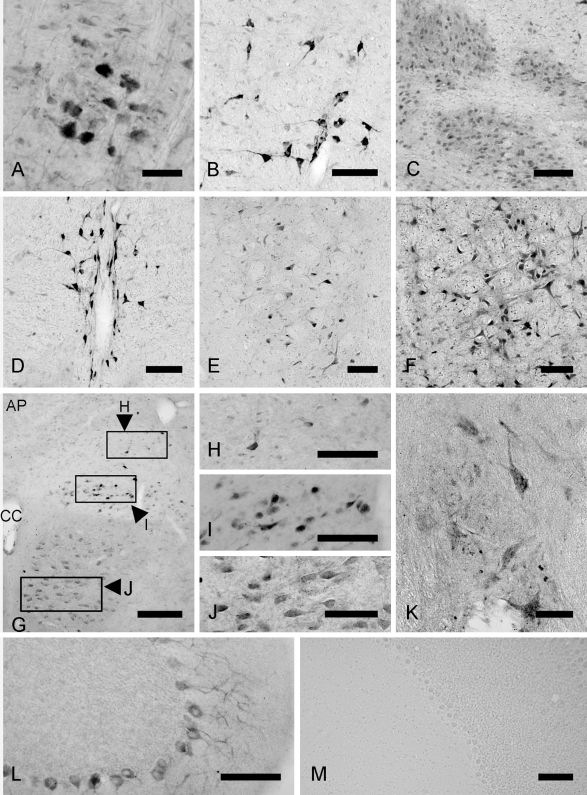
Fig. 3.
Immunohistochemical localization of TFG protein in the nuclei of medulla (A–J), the cerebellum (L), and the upper cervical spinal cord (K). A–J; In the medulla, TFG-positive neurons were seen in the ambiguus nucleus (A), the gigantocellular reticular nucleus (B), inferior olive (C), the raphe obscurus nucleus (D), the spinal trigeminal nucleus (E), the lateral reticular nucleus (F), the nucleus of the solitary tract (G and H), the dorsal motor nucleus of the vagus (G and I), and the hypoglossal nucleus (G and J). L and M; In the cerebellum, no signal was observed following preincubation peptide absorption (M). Purkinje cells showed immunostaining for TFG (L). K; In the spinal cord, large motor neurons in the anterior horn of the spinal cord were stained for TFG immunoreactivity (K). Bars=50 µm (A); 100 µm (B–F, H–M); 200 µm (G).
Distribution of TFG-positive neurons in the midbrain, pons, and medulla
The most prominent TFG immunoreactivity in the midbrain (Fig. 1A and 1B) was observed in the mesencephalic trigeminal nucleus (Fig. 2A) and the raphe nuclei (Fig. 2B and 2C). In the mesencephalic trigeminal nucleus, large neurons with paraphytes, named by Nageotte [2, 15], were intensely positive for TFG (Fig. 2A). In the dorsal (Fig. 2B and 2C) and median raphe nuclei (Fig. 2D), clusters of intensely stained neurons were observed. At a large magnification, TFG-immunoreactivity was localized to neuronal somata and proximal processes (Fig. 2C). In the oculomotor nucleus, large neurons were very faintly stained for TFG (Fig. 2E).
In the pons (Fig. 1C and 1D), densely packed, round, small neurons were TFG-immunopositive in the pontine nuclei (Fig. 2F). In the ventral cochlear nucleus, oval neurons showed moderate TFG immunoreactivity (Fig. 2G). In the nucleus of the abducens, large neurons were moderately stained for TFG (Fig. 2H and 2I), while moderate immunoreactivity was observed in many medium to large-sized neurons in the lateral vestibular nucleus (Fig. 2H and 2J). Oval neurons in the nucleus of the trapezoid body also showed moderate immunoreactivity (Fig. 2H and 2K). Some positive neurons were seen in the locus coeruleus (Fig. 1C). Although the neurons in the facial nucleus could not be seen, neurons in the perifacial zone were intensely stained for TFG (Fig. 2H and 2L).
In the medulla (Fig. 1E–G), motor neurons in the ambiguus nucleus were positive for TFG (Fig. 3A). Neurons intensely stained for TFG were scattered in the gigantocellular reticular nucleus (Fig. 3B). In these neurons, TFG-immunoreactivity was mainly localized to neuronal somata and proximal dendrites (Fig. 3B). Faint immunoreactivity was seen in densely packed, round, small neurons in the inferior olive (Fig. 3C). In the raphe obscurus of medulla, immunoreactive neurons spreading along the midline were apparent (Fig. 3D). Some neurons were positive for TFG in the spinal trigeminal nucleus (Fig. 3E) and the lateral reticular nucleus (Fig. 3F). Faint TFG immunostaining was also observed in some neurons at the rim of the nucleus of solitary tract (Fig. 3G and 3H). Neurons in the dorsal motor nucleus of the vagus showed two different immunoreactive intensities for TFG (Fig. 3G and 3I); those with intense immunoreactivity for TFG were wide cells with dendrites. TFG-positive immunoreactivity was also recognized in the hypoglossal nucleus (Fig. 3G and 3J).
Distribution of TFG-positive neurons in the cerebellum and the spinal cord
In the cerebellum, Purkinje cells were clearly positive for TFG, and were moderately stained (Fig. 3L). In the spinal cord, large motor neurons in the anterior horn were moderately immunostained for TFG throughout the spinal cord including the cervical (Fig. 3K, 4A and 4B) and lumbar spinal cord (Fig. 4C and 4D).
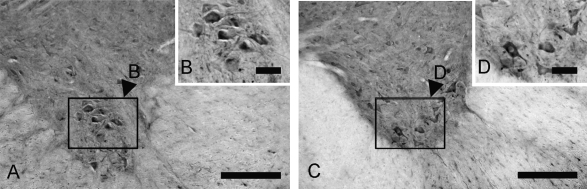
Fig. 4.
Immunohistochemical localization of TFG protein in the anterior horn of the cervical (A and B), and the lumbar (C and D) spinal cord. A and B; TFG-positive neurons seen in the anterior horn at the C3 cervical spinal cord (A). Magnified image of box in A (B). C and D; TFG-positive neurons seen in the anterior horn at the L2 lumbar spinal cord (C). Magnified image of box in C (D). Bars=200 µm (A and C); 50 µm (B and D).
Pre-incubation of the antibody with a TFG peptide removed any staining, which confirmed that the observed staining was specific for TFG (Fig. 3M).
TFG-positive neurons partly colocalize with serotonin-positive neurons in the dorsal raphe nucleus
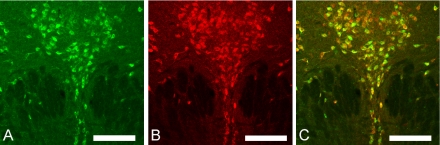
The distribution pattern of TFG-positive neurons was very similar to that of serotonin-positive neurons in the raphe nuclei (Fig. 2B–D, and 3D). Therefore, double immunofluorescent histochemical staining of rat midbrain sections was employed to investigate the cellular colocalization of TFG and serotonin (Fig. 5). The ratio of colocalization varied among the raphe subnuclei (Table 1). In the dorsal raphe nuclei, the ratio of colocalized neurons to total neurons were 28% (Table 1), where 44% of serotonin-positive neurons stained for TFG, and 42% of TFG-positive neurons were positive for serotonin. In the paramedian and median raphe nuclei, the ratios of TFG and serotonin colocalization were only 5% and 16%, respectively (Table 1).
Fig. 5.
Double immunostaining for TFG (green) and serotonin (red) in the dorsal raphe nucleus. A; TFG-immunoreactive neurons (A). B; serotonin-immunoreactive neurons (B). C; Merged image of A and B (C). Bars=200 µm.
Table 1.
Counts (and percentages) of neurons immunoreactive for TFG, serotonin or both in the raphe nuclei
| Total cell number | TFG (+)/serotonin (−) (%) | TFG (−)/serotonin (+) (%) | TFG (+)/serotonin (+) (%) | |
|---|---|---|---|---|
| Dorsal Raphe | 667 | 249 (37.3) | 234 (35.1) | 184 (27.6) |
| Paramedian Raphe | 151 | 130 (86.1) | 14 (9.3) | 7 (4.6) |
| Median Raphe | 179 | 69 (38.5) | 82 (45.8) | 28 (15.6) |
| Total | 997 | 448 (44.9) | 330 (33.1) | 219 (22.0) |
IV. Discussion
The present study demonstrated localization of TFG-positive neurons in the rat brainstem, cerebellum, and spinal cord using the antibody specific to TFG proteins. The production and characterization of the anti-TFG antibody were previously reported [11]. In brief, the antibody recognizes the common region of the conventional and variant forms of rat TFG. Western blot analysis using rat brain homogenates showed an intense band of about 50 kDa and a weak band of about 30 kDa, which are the expected sizes of the conventional and variant form of TFG, respectively. Immunohistochemical analysis of the rat brain has identified specific TFG-positive neuronal populations but the distribution patterns of these neurons were not reported. In this study, we mapped TFG-positive neurons in rat brainstem, cerebellum, and spinal cord using the TFG-specific antibody. Knowledge of the distribution patterns of TFG will facilitate further analyses of the functional role of TFG in neurons.
The most conspicuous result in this study is that neurons intensely stained for TFG were distributed in the raphe nuclei in the brainstem. The distribution pattern of TFG in the raphe nuclei was similar to that of serotonin neurons. Double immunostaining for TFG and serotonin demonstrated that TFG was localized to a subpopulation of serotonin. In addition, some TFG-positive neurons were negative for serotonin.
We demonstrated that TFG is mainly localized in the cytoplasm of neurons, and shows an immunostaining pattern consistent with a recent study that localized TFG to endoplasmic reticulum exit sites [23]. TFG has been suggested to play an important role in vesicle transportation from the endoplasmic reticulum to the Golgi [23]. Interestingly, we demonstrated TFG immunoreactivity in proximal processes and varicose fibers in some neurons, suggesting a novel function of TFG that differs from its role at the endoplasmic reticulum. There was only weak immunostaining of TFG in axons and axonal terminals with the TFG antibody we used in this study, suggesting that TFG is probably not involved in axonal extension. Interestingly, TFG-positive neurons were observed in specific brain regions, and we have mapped these regions in the rat brainstem (Fig. 1).
TFG-positive neurons were found in some cranial nuclei-containing motor neurons, although not in all motor neurons. Additionally, TFG immunoreactivity was found in the anterior horn motor neurons throughout the spinal cord. These findings indicate that TFG could play a role in motor movement or motor coordination. The additional observation of TFG immunoreactivity in Purkinje cells in the cerebellum argues for a role in motor coordination. To examine the physiological role of TFG in the nervous system, further study using animal models will be needed.
V. Conclusion
We have mapped TFG-positive neurons in the rat brainstem, cerebellum, and spinal cord. In the brainstem, neurons intensely positive for TFG were distributed in the raphe nuclei, the gigantocellular reticular nucleus, the reticulotegmental nucleus of the pons, and some cranial nerve nuclei such as the trigeminal nuclei, the vestibulocochlear nuclei, and the vagus nerve nuclei. Purkinje cells in the cerebellum and motor neurons in the spinal anterior horn were also TFG-positive. The TFG immunoreactive neurons in the raphe nuclei were colocalized to a subpopulation of serotonergic neurons. These localization data provide the basis for further studies of the functional role of TFG in the brain.
VI. Acknowledgments
This study was supported by Grants-in-Aid from the Ministry of Education, Culture, Science, Sports and Technology of Japan. We thank members of the laboratory who helped and gave suggestions on the brain mapping. We thank T. Yamamoto of the Central Research Laboratory at Shiga University of Medical Science for his technical assistance with the microscopic images. In addition, we thank H. Satoh and M. Oshima for their help with the immunohistochemistry.
VII. References
- 1.Abe H., Tooyama I., Reanda T., Erspamer V., Kimura H. Immunohistochemical demonstration of [D-Ala2]deltorphin-I in amacrine cells of rat retina. Peptides. 1994;15:49–54. doi: 10.1016/0196-9781(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 2.Card J. P., Riley J. N., Moore R. Y. The motor trigeminal nucleus of the rat: analysis of neuronal structure and the synaptic organization of noradrenergic afferents. J. Comp. Neurol. 1986;250:469–484. doi: 10.1002/cne.902500406. [DOI] [PubMed] [Google Scholar]
- 3.Chase A., Ernst T., Fiebig A., Collins A., Grand F., Erben P., Reiter A., Schreiber S., Cross N. C. TFG, a target of chromosome translocations in lymphoma and soft tissue tumors, fuses to GPR128 in healthy individuals. Haematol. 2010;95:20–26. doi: 10.3324/haematol.2009.011536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen L., McCloskey T., Joshi P. M., Rothman J. H. ced-4 and proto-oncogene tfg-1 antagonistically regulate cell size and apoptosis in C. elegans. Curr. Biol. 2008;18:1025–1033. doi: 10.1016/j.cub.2008.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greco A., Fusetti L., Miranda C., Villa R., Zanotti S., Pagliardini S., Pierotti M. A. Role of the TFG N-terminus and coiled-coil domain in the transforming activity of the thyroid TRK-T3 oncogene. Oncogene. 1998;16:809–816. doi: 10.1038/sj.onc.1201596. [DOI] [PubMed] [Google Scholar]
- 6.Hernandez L., Pinyol M., Hernandez S., Bea S., Pulford K., Rosenwald A., Lamant L., Falini B., Ott G., Mason D. Y., Delsol G., Campo E. TRK-fused gene (TFG) is a new partner of ALK in anaplastic large cell lymphoma producing two structurally different TFG-ALK translocations. Blood. 1999;94:3265–3268. [PubMed] [Google Scholar]
- 7.Hisaoka M., Ishida T., Imamura T., Hashimoto H.2004TFG is a novel fusion partner of NOR1 in extraskeletal myxoid chondrosarcoma. Genes Chromosomes Canc. 40325–328. [DOI] [PubMed] [Google Scholar]
- 8.Horvat A., Schwaiger F., Hager G., Brocker F., Streif R., Knyazev P., Ullrich A., Kreutzberg G. W. A novel role for protein tyrosine phosphatase shp1 in controlling glial activation in the normal and injured nervous system. J. Neurosci. 2001;21:865–874. doi: 10.1523/JNEUROSCI.21-03-00865.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaplan D. R., Hampstead B. L., Martin-Zanca D., Chao M. V., Parada L. F. The trk proto-oncogene product: a signal transducing receptor for nerve growth factor. Science. 1991;252:554–558. doi: 10.1126/science.1850549. [DOI] [PubMed] [Google Scholar]
- 10.Klein R., Jing S. Q., Nanduri V., O’Rourke E., Barbacid M. The trk proto-oncogene encodes a receptor for nerve growth factor. Cell. 1991;65:189–197. doi: 10.1016/0092-8674(91)90419-y. [DOI] [PubMed] [Google Scholar]
- 11.Maebayashi H., Takeuchi S., Masuda C., Makino S., Fukui K., Kimura H., Tooyama I. Expression and localization of TRK-fused gene products in the rat brain and retina. Acta Histochem. Cytochem. 2012 doi: 10.1267/ahc.11015. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Massa P. T., Saha S., Wu C., Jarosinski K. W. Expression and function of the protein tyrosine phosphatase SHP-1 in oligodendrocytes. Glia. 2000;29:376–385. [PubMed] [Google Scholar]
- 13.Mencinger M., Panagopoulos I., Andreasson P., Lassen C., Mitelman F., Aman P. Characterization and chromosomal mapping of the human TFG gene involved in thyroid carcinoma. Genomics. 1997;41:327–331. doi: 10.1006/geno.1997.4625. [DOI] [PubMed] [Google Scholar]
- 14.Mencinger M., Aman P. Characterization of TFG in Mus musculus and Caenorhabditis elegans. Biochem. Biophys. Res. Commun. 1999;257:67–73. doi: 10.1006/bbrc.1999.0417. [DOI] [PubMed] [Google Scholar]
- 15.Nageotte J. Etude sur la greffe des ganglions rachidiens; variations et tropismes du neurone sensitif. Anat. Anz. 1907;31:225–245. [Google Scholar]
- 16.Nakahara H., Konishi Y., Beach T. G., Yamada N., Makino S., Tooyama I. Infiltration of T-lymphocytes and expression of ICAM-1 in the hippocampus of patients with hippocampal sclerosis. Acta Histochem. Cytochem. 2010;43:157–162. doi: 10.1267/ahc.10022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okano H., Bamba H., Hisa Y., Makino S., Ando S., Tamiya G., Goto S., Kaji R., Kimura H., Tooyama I. Immunohistochemical study of TAFII250 in the rat laryngeal nervous system. Histol. Histopathol. 2005;20:1029–1035. doi: 10.14670/HH-20.1029. [DOI] [PubMed] [Google Scholar]
- 18.Paxinos G., Watson C. The Rat Brain in Stereotaxic Coodinates 2nd ed. Academic Press; San Diego; 1986. [Google Scholar]
- 19.Plutzky J., Neel B. G., Rosenberg R. D. Isolation of a src homology 2-containing tyrosine phosphatase. Proc. Natl. Acad. Sci. U S A. 1992;89:1123–1127. doi: 10.1073/pnas.89.3.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roccato E., Pagliardini S., Cleris L., Canevari S., Formelli F., Pierotti M. A., Greco A. Role of TFG sequences outside the coiled-coil domain in TRK-T3 oncogenic activation. Oncogene. 2003;22:807–818. doi: 10.1038/sj.onc.1206189. [DOI] [PubMed] [Google Scholar]
- 21.Roccato E., Miranda C., Raho G., Pagliardini S., Pierotti M. A., Greco A. Analysis of SHP-1-mediated down-regulation of the TRK-T3 oncoprotein identifies Trk-fused gene (TFG) as a novel SHP-1-interacting protein. J. Biol. Chem. 2005;280:3382–3389. doi: 10.1074/jbc.M407522200. [DOI] [PubMed] [Google Scholar]
- 22.Tohyama I., Kameyama M., Kimura H. Quantitative morphometric analysis of two types of serotonin-immunoreactive nerve fibres differentially responding to p-chlorophenylalanine treatment in the rat brain. Neurosci. 1988;26:971–991. doi: 10.1016/0306-4522(88)90113-3. [DOI] [PubMed] [Google Scholar]
- 23.Witte K., Schuh A. L., Hegermann J., Sarkeshik A., Mayers J. R., Schwarze K., Yates J. R., 3rd., Eimer S., Audhya A. TFG-1 function in protein secretion and oncogenesis. Nat. Cell Biol. 2011;13:550–558. doi: 10.1038/ncb2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu C., Sun M., Liu L., Zhou G. W. The function of the protein tyrosine phosphatase SHP-1 in cancer. Gene. 2003;306:1–12. doi: 10.1016/s0378-1119(03)00400-1. [DOI] [PubMed] [Google Scholar]
- 25.Yasuhara O., Tooyama I., Aimi Y., Bellier J. P., Hisano T., Matsuo A., Park M., Kimura H. Demonstration of cholinergic ganglion cells in rat retina: expression of an alternative splice variant of choline acetyltransferase. J. Neurosci. 2003;23:2872–2881. doi: 10.1523/JNEUROSCI.23-07-02872.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]