Abstract
Many patients with advanced genitourinary malignancies develop bone metastases, which can lead to potentially debilitating skeletal complications. Moreover, age-related bone loss and cancer treatments such as hormonal therapy for prostate cancer can weaken bone, placing patients at risk for osteoporotic fractures in addition to skeletal-related events (SREs) from bone metastases. Zoledronic acid, a bisphosphonate, is approved worldwide to reduce the risk of SREs in patients with bone metastases from solid tumors or bone lesions from multiple myeloma. Zoledronic acid, although underutilized in genitourinary malignancies, has long been the mainstay of treatment in patients with bone metastases, and can also help preserve bone during anticancer therapy. Recently, denosumab, a monoclonal antibody directed against the receptor activator of nuclear factor kappa-B ligand, was approved in the United States and the European Union for reducing the risk of SREs in patients with bone metastases from solid tumors. Denosumab (at a lower dose) is also approved in the European Union and the United States to treat androgen deprivation-induced bone loss in men with prostate cancer. In addition, preclinical rationale and emerging clinical data suggest that bone-modifying agents may be able to delay disease progression in genitourinary cancers, just as newly developed anticancer treatments have produced reductions in SREs, possibly by indirect effects on the disease course. This review article summarizes current data and ongoing studies to preserve bone health in patients with advanced genitourinary cancers.
Keywords: bisphosphonate, bone metastases, genitourinary cancer, prostate cancer, zoledronic acid
Introduction
Genitourinary malignancies: burden of disease and challenges to bone health
Prostate cancer (PC) is the most commonly diagnosed solid tumor in men (approximately 800,000 new cases diagnosed worldwide every year) [Garcia et al. 2007], and approximately three of every four patients with PC develop bone metastases, often at first diagnosis of metastatic disease [Coleman, 2001]. Less prevalent genitourinary (GU) malignancies also have a predilection for metastasis to the skeleton: bone metastases have been reported in 20–40% of patients with stage IV renal cell carcinoma (RCC) or bladder cancer [Coleman, 2001]. Bone metastases can disrupt normal bone homeostasis (characterized by balanced and spatially coupled interactions between osteoclasts [bone resorption] and osteoblasts [bone formation] that are responsible for normal bone maintenance and repair), thereby weakening the skeleton [Coleman, 2001]. Radiographically, bone lesions from GU cancers may appear to be predominantly osteolytic, osteoblastic, or can have mixed characteristics. Nonetheless, all bone lesions are associated with elevated bone turnover levels, and such elevated osteoclast activity can release tumor-stimulating growth factors from the bone [Mundy, 2002]. The resulting vicious cycle of cancer growth and bone destruction can lead to skeletal-related events (SREs), including pathologic fractures, spinal cord compression, the need for palliative radiotherapy, surgery to bone, cementoplasty, and hypercalcemia of malignancy [Coleman, 2001]. Without bone-directed treatment, the majority of patients with advanced PC will experience an SRE during the course of their disease [Saad et al. 2004]. Moreover, in retrospective analyses of a 21-month, randomized, phase III, placebo-controlled trial in patients with bone metastases from solid tumors (excluding breast or prostate cancer) [Rosen et al. 2004], approximately 75% of patients in the RCC subset who did not receive bisphosphonate (BP) therapy developed an SRE [Saad and Lipton, 2005].
Even before the development of bone metastases, bone health may be compromised in patients with GU cancers because of age-related osteoporosis and the detrimental effects of anticancer therapies on bone mineral density (BMD). This is most evident in the PC setting, wherein surgical or hormonal castration (androgen-deprivation therapy [ADT]) to lower testosterone levels is common in patients who have high-risk disease, or whose prostate-specific antigen levels remain elevated or continue to increase after primary locoregional treatment [Aus et al. 2005; Heidenreich et al. 2009]. Because ADT is associated with rapid bone loss, it places patients at a greatly increased risk for fractures [Mittan et al. 2002; National Comprehensive Cancer Network, 2009; Preston et al. 2002; Smith, 2006]. Indeed, because PC is especially prevalent among elderly men, many patients have low BMD even before initiating ADT [Daniell et al. 2000], making them especially vulnerable to the effects of ADT-associated bone loss. Overall, men with early PC face early challenges to bone health and may require bone-directed therapy to preserve BMD and reduce fracture risk.
Bone-modifying agents for advanced GU malignancies
Bisphosphonates are antiresorptive agents that block pathologic bone resorption by inhibiting osteoclast activation and function [Boyle et al. 2003; Rogers et al. 2000], and have been the standard of care for maintaining bone health in patients with bone metastases from solid tumors and bone lesions from multiple myeloma for more than a decade [Aapro et al. 2008]. Bisphosphonate therapy interrupts the vicious cycle of increased osteolysis coupled with increased tumor growth, thereby preserving bone health and potentially delaying bone lesion progression [Mundy, 2002]. Several BPs have been evaluated in patients with bone metastases from GU malignancies [Donat et al. 2006; Ernst et al. 2003; Hatoum et al. 2008, 2011; Hering et al. 2003; Lipton et al. 2002; Rodrigues et al. 2004; Saad, 2008; Saad et al. 2004]; although both oral and intravenous BPs have shown palliative activity in these settings, zoledronic acid (ZOL) is the only BP to have demonstrated significant objective and durable benefits and to have received broad regulatory approval for preventing SREs in patients with bone metastases from castration-resistant PC (CRPC) or other GU cancers (e.g. RCC and bladder cancer) [Lipton et al. 2003; Saad et al. 2002, 2004; Zaghloul et al. 2010]. Denosumab, a monoclonal antibody against the receptor activator of nuclear factor kappa-B ligand (RANKL), received regulatory approval in the United States in 2010 and in the European Union in July 2011 [Amgen Inc., 2011] for preventing SREs in patients with bone metastases from solid tumors, but not multiple myeloma [Amgen Inc., 2010]. Denosumab (at a lower dose and treatment frequency compared with advanced oncology settings) is also approved in the United States and the European Union to treat postmenopausal osteoporosis in women at high risk for fracture, and to treat bone loss associated with hormonal therapy for PC in men at high risk for fracture (defined as age >70 years, osteopenia, or history of osteoporotic fracture) [Amgen Europe B.V., 2010]. Although the mechanisms of action of denosumab and BPs are different, both classes of agents inhibit pathologic bone turnover, resulting in reduced skeletal morbidity.
In addition to their established role for preserving skeletal integrity in patients with malignant bone disease, bone-modifying agents also help preserve BMD and can reduce fracture risk during cancer treatment, most notably during ADT for PC [Bhoopalam et al. 2009; Casey et al. 2010; Greenspan et al. 2007; Israeli et al. 2007; Izumi et al. 2009; Planas et al. 2009; Ryan et al. 2006; Smith et al. 2001, 2003, 2009; Taxel et al. 2010]. Emerging evidence also suggests that bone-modifying agents may delay the progression of bone lesions and help delay the development of skeletal and other metastases [Lipton et al. 2003, 2008; Smith et al. 2012; Zaghloul et al. 2010], potentially through making the bone microenvironment less conducive to tumor growth. The ‘seed and soil’ hypothesis provides an excellent theoretical framework for understanding the predilection of cancer cells for bone [Paget, 1889], and describes the skeleton as providing a fertile ‘soil’ for the germination and growth of cancer ‘seeds’. Circulating tumor cells (CTCs) may act as ‘seeds’ for subsequent recurrences in supportive ‘soil’, either at the primary tumor site (tumor ‘self-seeding’) or in distant sites (e.g. bone or visceral organs such as the liver) [Mundy, 2002; Norton and Massague, 2006]. It is also becoming evident that the bone marrow microenvironment can harbor CTCs (referred to as disseminated tumor cells [DTCs] when detected via bone marrow biopsy) and provide a secure niche allowing DTCs to survive for prolonged periods of time and evade the cytotoxic or proapoptotic effects of systemic anticancer therapies [Clines and Guise, 2008; Meads et al. 2008; Mundy, 2002; Shiozawa et al. 2008]. Furthermore, persistence of not only CTCs but also DTCs despite anticancer therapies has been associated with increased risks of recurrence and distant metastases in patients with PC [Anand et al. 2010; Berg et al. 2007; Danila et al. 2007, 2010; Kollermann et al. 2008; Lee et al. 2009; Morgan et al. 2009; Olmos et al. 2009; Weckermann et al. 2009], further supporting the concept that manipulating the bone microenvironment to make it less supportive of DTC survival might provide a means to prevent or delay the development of overt metastases.
We discuss below current evidence supporting the use of ZOL in patients with advanced GU cancers to reduce the risk of SREs, and provide perspective on recent advances supporting a role for bone-directed therapies to delay disease progression in this setting.
Established benefits of bone-modifying agents in advanced GU malignancies
Castration-resistant prostate cancer
Zoledronic acid is the only BP that has demonstrated significant objective long-term (2-year) efficacy in patients with bone metastases from CRPC and has achieved widespread regulatory approval in this setting. Although the majority of current guidelines for BP therapy in PC clearly recommend ZOL, some may include other BPs, or even recommend BPs in general without any qualifications [British Association of Urological Surgeons, 2005; Heidenreich et al. 2009; National Comprehensive Cancer Network, 2009]. It is therefore important to distinguish between BP use to preserve BMD during ADT or palliate pain from PC bone metastases and BP therapy to reduce the risk of SREs in patients with advanced CRPC. Zoledronic acid is the only BP approved for the latter purpose, although several BPs have demonstrated palliative benefits in patients with bone metastases from PC (Table 1) [Donat et al. 2006; Ernst et al. 2003; Heidenreich et al. 2005; Hering et al. 2003; Rodrigues et al. 2004; Saad and Eastham, 2010a; Saad et al. 2004; Small et al. 2003].
Table 1.
Summary of trials with bisphosphonates for reducing bone pain in prostate cancer.
| Trial | Treatment | Dose and regimen | Pain measurement | Results | p value |
|---|---|---|---|---|---|
| Zoledronic acid | |||||
| Randomized, double-blind study in metastatic prostate cancer (N=422) [Saad et al. 2004] | Zoledronic acid, placebo | 4 mg IV every 3 weeks for 2 years | BPI | Significantly reduced mean composite BPI compared with placebo at 24 months | 0.024 |
| Clodronate | |||||
| Randomized, double-blind study in metastatic prostate cancer (N=209) [Ernst et al. 2003] | Clodronate | 1,500 mg IV every 3 weeks | PPI (0–5 scale) | Reduced pain scores | NS |
| Open-label, single-arm study in prostate cancer (N=58) [Rodrigues et al. 2004] | Clodronate | IV every 28 days | Visual pain scale (0–10) | Average improved from 7.4 to 2.4 over 3 years | NR |
| Open-label, single-arm study in prostate cancer (N=32) [Hering et al. 2003] | Clodronate | IV every 28 days | Visual pain scale (0–10) | Average improved from 7.7 to 2.1 over 2 years | NR |
| Open-label, single-arm study in prostate cancer (N=78) [Donat et al. 2006] | Clodronate | Oral 800 mg/day | Visual pain scale (0–10) | Reduced pain scores | NR |
| Pamidronate | |||||
| Pooled analysis of 2 randomized, double-blind studies in prostate cancer (N=378) [Small et al. 2003] | Pamidronate versus placebo | 90 mg IV every 3 weeks | BPI | Reduced pain score | NS |
| Ibandronate | |||||
| Phase II study in prostate cancer (n=45) [Heidenreich et al. 2005] | Ibandronate | 6 mg IV for 3 consecutive days, then 6 mg IV every 4 weeks | Mean VAS (0–10 points) | Reduced pain score | NR |
Abbreviations: BPI, Brief Pain Inventory; IV, intravenous; NR, not reported; NS, not significant; PPI, present pain intensity scale of the McGill–Melzack Pain Questionnaire; VAS, Visual Analogue Scale.
Reprinted from Semin Oncol, 37(Suppl. 1), Saad and Eastham, Maintaining bone health in prostate cancer throughout the disease continuum, S30-S37, Copyright 2010, with permission from Elsevier [2010a].
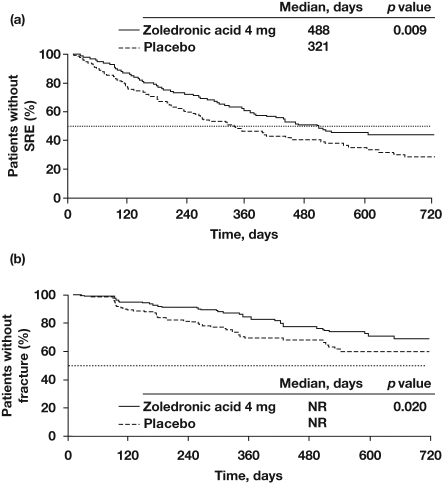
In a 2-year, randomized, double-blind trial in patients with bone metastases from CRPC, ZOL (4 mg via 15-minute infusion every 3 weeks) significantly reduced the proportion of patients with an on-study SRE versus placebo (38% versus 49%; p = 0.028; in 122 patients who completed 24 months on study), delayed the median time to first SRE by almost 6 months (Figure 1a) [Saad and Lipton, 2005], and reduced the ongoing risk of SREs by 36% (p < 0.01) [Saad and Lipton, 2005]. Although not statistically significant, patients receiving ZOL had a 2.6-month improvement in overall survival (OS) compared with placebo (p = 0.103) [Saad and Lipton, 2005]. In analyses of the initial 15-month core phase of this trial (N = 643), ZOL (4 mg) not only reduced the risk of any SREs but also significantly reduced the incidence and delayed the onset of pathologic fracture, an SRE associated with high morbidity and increased mortality, versus placebo [Saad et al. 2007] (p = 0.020; Figure 1b) [Saad and Lipton, 2005]. Moreover, benefits were seen early in the study; ZOL significantly decreased the proportion of patients with an SRE within the first 3 months compared with placebo [Lipton et al. 2002].
Figure 1.
Zoledronic acid (4 mg every 3–4 weeks) delayed the first on-study skeletal-related event (SRE) (a) and pathologic fracture (b) compared with placebo in patients with bone metastases from castration-resistant prostate cancer. Reproduced with permission from Saad and Lipton [2005]. © 2005 John Wiley and Sons.
In a phase III, head-to-head trial versus ZOL in patients with bone metastases from CRPC, denosumab demonstrated non-inferiority to ZOL for delaying the first on-study SRE (hazard ratio [HR] = 0.82; p = 0.0002) [Fizazi et al. 2011]. Denosumab also met the secondary endpoint of superiority for delaying the first on-study SRE versus ZOL (p = 0.008) [Fizazi et al. 2011]. These data formed the basis for the approval of denosumab for treating patients with bone metastases from CRPC [Amgen Inc., 2010], thereby expanding the therapeutic repertoire in this setting.
In addition to classic bone-directed therapies, abiraterone acetate, an inhibitor of androgen biosynthesis, is showing promising signs for skeletal health benefits, most likely as a consequence of its effects on overall disease progression. In a phase III trial in 1195 men previously treated with docetaxel for CRPC, abiraterone acetate improved OS versus placebo (HR = 0.65; p < 0.001) [de Bono et al. 2011]. In other analyses from this trial, abiraterone acetate also prolonged the time to first on-study SRE versus placebo (p = 0.0006) [Logothetis et al. 2011]. Abiraterone acetate reduces androgen levels and is administered in combination with low-dose prednisone [de Bono et al. 2011], two factors that increase the potential for BMD decreases during long-term therapy. Although the long-term effects of abiraterone acetate plus prednisone treatment on BMD are as yet unknown, it is possible that concurrent antiresorptive therapy might be needed in some patients. Longer follow up and future studies may provide insight into the possible requirements for combination therapies.
In addition to revealing the potential for SRE-reduction activity, the phase III trial of abiraterone acetate also provided important insights into the prognostic role of CTCs and the effects of treatment on CTC levels [Scher et al. 2011]. In patients with unfavorable CTC status at baseline (defined as ≥5 CTCs/7.5 ml blood), 48% of the abiraterone acetate-treated patients transitioned to favorable CTC status (<5 CTCs/7.5 ml blood) by week 12, compared with only 17% of placebo-treated patients (p < 0.0001) [Scher et al. 2011]. Conversion in CTC status versus baseline was predictive of OS as early as 4 weeks after initiating treatment, and appeared to be a promising predictive marker for OS in this trial [Scher et al. 2011]. A planned study in patients with bone metastases from prostate or renal cancers who are receiving ZOL treatment will further explore the prognostic value of CTCs and the potential effects of ZOL on CTC levels and persistence; registration of this protocol and initiation of patient accrual are expected in the near future. Outcomes from this trial are expected to provide important insights into the value of CTC assessments in patients with advanced disease receiving bone-modifying therapies.
Renal cell carcinoma and bladder cancer
Little guidance has been published regarding the utility of bone-modifying agents in patients with RCC and bladder cancer. Nonetheless, current data support the use of BPs to prevent SREs and preserve quality of life and functional autonomy in patients with bone metastases. In the phase III trial of ZOL in patients with bone metastases from lung cancer and other solid tumors (N = 773; N = 507 excluding the ZOL 8-mg/4-mg arm), subsets of patients had RCC (n = 46) or bladder cancer (n = 26) [Lipton et al. 2003; Rosen et al. 2003]. Retrospective subset analyses showed that ZOL reduced the proportion of patients with 1 or more SREs and prolonged the time to first SRE compared with placebo in both the RCC and bladder cancer cohorts (Table 2) [Abrahamsson et al. 2008; Lipton et al. 2003; Saad and Eastham, 2010b; Saad and Lipton, 2005; Zaghloul et al. 2010]. In additional retrospective analyses, ZOL was found to reduce the skeletal morbidity rate, reduce the risk of developing an SRE, and delay the time to first pathologic fracture compared with placebo among RCC patients (Table 2) [Abrahamsson et al. 2008; Lipton et al. 2003; Saad and Eastham, 2010b; Saad and Lipton, 2005; Zaghloul et al. 2010]. In the RCC subset from the ZOL pivotal trial (n = 46), treatment with 4 mg ZOL every 3 weeks significantly reduced the proportion of patients with on-study SREs (37% versus 74% with placebo; p = 0.015) [Lipton et al. 2003; Rosen et al. 2003]. This was accompanied by reductions in the skeletal morbidity rate (2.68 SREs/year with ZOL versus 3.38 SREs/year with placebo) and in the cumulative risk of developing SREs (61% risk reduction; p = 0.008) [Lipton et al. 2003; Rosen et al. 2003]. Furthermore, ZOL has been shown to reduce bone pain in an observational study in patients with malignant bone disease (N = 472) receiving monthly ZOL treatment for up to 24 months [Abrahamsson et al. 2008]. In this study, greater than 50% of patients with prostate cancer or RCC (combined n = 279) reported stable or reduced pain scores on the Visual Analog Scale (without increased analgesic usage) within 6 months of initiating ZOL treatment [Abrahamsson et al. 2008].
Table 2.
Effects of zoledronic acid versus placebo on skeletal-related events (SREs) in patients with renal cell carcinoma or bladder cancer.
| Endpoint | Renal cell carcinoma [Abrahamsson et al. 2008; Lipton et al. 2003; Saad and Lipton, 2005] | Bladder cancer [Zaghloul et al. 2010] |
|---|---|---|
| Reduced proportion of patients with ≥1 SRE | 41% versus 79%; p=0.011 | 65% versus 90%; p=0.010 |
| Reduced mean number of SREs | NR | 0.95 versus 2.05; p=0.001 |
| Prolonged median time to first SRE | 424 versus 72 days; p=0.007 | 112 versus 56 days; p=0.0001 |
| Reduced skeletal morbidity rate | 2.58 versus 3.13; p=0.009 | NR |
| Reduced risk of developing an SRE | 58% (HR=0.418; p=0.010) | 50% (HR=0.413; p=0.008) |
| Delayed time to first pathologic fracture | Not reached versus 168 days; p=0.003 | NR |
| Reduced bone pain score | 20.0 versus 37.3 units; p=NR | 2.95 versus 4.37 units; p=0.015 |
Abbreviations: HR, hazard ratio; NR, not reported.
Reproduced from Semin Oncol, 37(Suppl. 1), Saad and Eastham, Zoledronic acid use in patients with bone metastases from renal cell carcinoma or bladder cancer, S38-S44, Copyright 2010, with permission from Elsevier [2010b].
Although the pivotal trial of ZOL predates the use of antiangiogenic therapies in RCC, observational studies of ZOL in combination with the newer treatment modalities for RCC have shown promising results. For example, a case report recently showed rapid reduction in metastatic bone lesions within 4–6 weeks and improvement in quality of life in a patient with RCC after receiving radiotherapy in combination with ZOL and sunitinib [Hird et al. 2008]. An additional case report on a patient with bone and visceral metastases from RCC revealed that combination therapy with ZOL plus interferon alpha reduced tumor size and prevented progression of metastases [Miwa et al. 2009]. Moreover, in a retrospective study of 23 patients with RCC, ZOL in combination with radiotherapy elicited a higher response rate (six patients versus one patient; p = 0.019), reduced the proportion of patients with an SRE (one patient versus 10 patients; p = 0.003), and prolonged SRE-free survival (not reached versus 18.7 months; p = 0.046) compared with radiotherapy alone [Kijima et al. 2009].
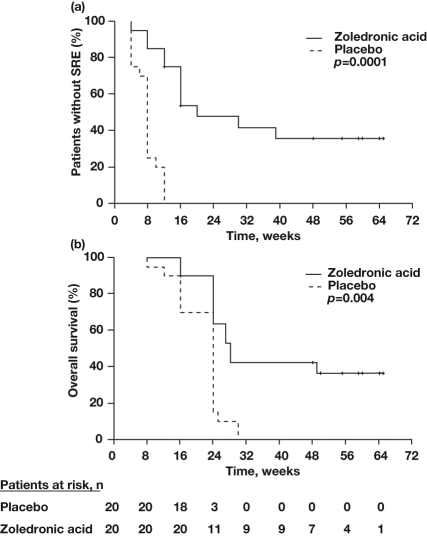
Similar to the situation with RCC, there is underappreciation of the importance of treating bone metastases in patients with bladder cancer and little published guidance for the use of BPs in this setting. As with RCC, ZOL has demonstrated SRE-reduction efficacy in patients with bone metastases from bladder cancer (Table 2) [Abrahamsson et al. 2008; Lipton et al. 2003; Saad and Eastham, 2010b; Saad and Lipton, 2005; Zaghloul et al. 2010]. Among the 773 patients enrolled in the phase III study of ZOL in lung cancer and other solid tumors, 26 patients had bone metastases from bladder cancer [Rosen et al. 2004]. Retrospective analysis of the subset of patients with advanced bladder cancer showed that ZOL reduced the risk of any SRE compared with placebo (33% versus 41%, respectively); however, small patient numbers precluded results from being statistically significant [Mulders et al. 2007]. The effect of ZOL on SREs has also been evaluated in a prospective, placebo-controlled, randomized study of ZOL in patients with bone metastases from bladder cancer (N = 40) [Zaghloul et al. 2010]. In this study, ZOL significantly reduced the mean number of SREs, overall SRE risk, and the proportion of patients with at least one SRE compared with placebo [Zaghloul et al. 2010]. In addition, ZOL reduced mean pain scores compared with placebo. Treatment with ZOL versus placebo also prolonged not only SRE-free survival (Figure 2a), but also OS (Figure 2b), suggesting a possible anticancer benefit with ZOL [Zaghloul et al. 2010]. Immunotherapy is used in both RCC and bladder cancer [Babjuk et al. 2011; Molina and Motzer, 2011], and the immunomodulatory effects of ZOL (especially activation of gamma-delta T cells) [Naoe et al. 2010] might prove beneficial in these settings.
Figure 2.
Zoledronic acid improved skeletal-related event (SRE)-free survival (a) and overall survival (b) compared with placebo in patients with bone metastases from bladder cancer. Reproduced with permission from Zaghloul et al. [2010].
As with the phase III trial of ZOL in patients with aggressive solid tumors, the phase III trial of denosumab versus ZOL in patients with multiple myeloma or bone metastases from solid tumors other than breast and prostate cancers included small numbers of patients with RCC (n = 155) and bladder cancer (n = 63) [Henry et al. 2011]. Denosumab was noninferior to ZOL for reducing the risk of SREs in the overall trial population [Henry et al. 2011]; however, specific subset data in patients with GU malignancies are yet to be reported.
Safety and tolerability of antiresorptive agents in patients with advanced GU cancers
Zoledronic acid
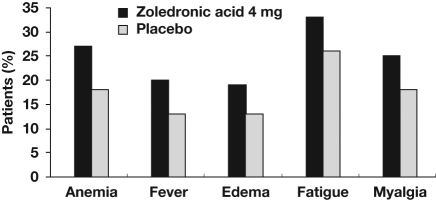
In phase III trials in patients with CRPC or RCC, ZOL was generally well tolerated compared with placebo [Lipton et al. 2003; Saad et al. 2004]. In the CRPC trial, the most frequent adverse events (AEs) reported with ZOL were fatigue, anemia, myalgia, and fever (Figure 3) [Parker, 2005]. The AE profile of ZOL in patients with RCC was similar: bone pain, pyrexia, and arthralgia were among the most frequently reported AEs in the ZOL and placebo groups [Lipton et al. 2003].
Figure 3.
Most frequently reported adverse events in the phase III placebo-controlled trial of zoledronic acid (4 mg every 3–4 weeks) in men with bone metastases from castration-resistant prostate cancer. Adapted with permission from Parker [2005]. © 2005 John Wiley and Sons.
All intravenous BPs are associated with dose- and infusion-rate-dependent effects on renal function. In the phase III trials of ZOL, the original 5-minute infusion in a 50-ml volume was amended to an infusion over at least 15 minutes in a 100-ml volume to ensure renal safety [Rosen et al. 2004]. The prescribing information for ZOL also includes guidelines for renal function monitoring and dose adjustment for baseline renal impairment in patients initiating ZOL treatment [Novartis Pharmaceuticals Corporation, 2011]. These dose adjustments are designed to maintain overall drug exposure to ZOL, as renal impairment slows the clearance rate of ZOL. Because GU malignancies, especially CRPC, are most common in elderly patients, in whom renal impairment is common, renal safety is an important consideration [Aapro and Launay-Vacher, 2011]. It has become evident that renal impairment (renal function outside normal boundaries) and renal insufficiency (more severe renal impairment) are common among patients with advanced cancers. In the Renal Insufficiency and Anticancer Medications (IRMA) study (N = 4684 patients with solid tumors including breast, colorectal, lung, ovarian, and prostate cancers), more than half of all patients had some level of abnormal renal function or renal insufficiency [Launay-Vacher et al. 2007]. In subset analyses by cancer type, approximately 63% of patients with PC (n = 222) had renal insufficiency, and 83% of 228 anticancer drug prescriptions required dose adjustments based on renal function [Launay-Vacher et al. 2009a]. In other subgroup analyses, 65% of all elderly patients enrolled in the IRMA study (n = 1553) had renal insufficiency, and the incidence of renal insufficiency increased with age [Launay-Vacher et al. 2009b]. Thus, adherence to the prescribing guidelines for ZOL is important to ensure renal tolerability, especially in patients at increased risk for renal impairment because of polypharmacy and age. In clinical practice, consistent use of these monitoring and dosing guidelines helps optimize patient safety during treatment with BPs and other agents.
Osteonecrosis of the jaw (ONJ) is an uncommon AE of multifactorial etiology, which has been described in patients with advanced cancer receiving complex treatment regimens including bone-modifying agents [Fizazi et al. 2011; Henry et al. 2011; Hoff et al. 2008]. As our understanding of the risk factors and course of ONJ has evolved, it is now evident that prophylactic dental care can reduce the risk of ONJ [Ripamonti et al. 2009] and that the majority of ONJ cases resolve with conservative treatment.
Denosumab
In the phase III head-to-head trials versus ZOL in patients with solid tumors other than breast cancer, rates of renal AEs were similar in the denosumab and ZOL treatment arms [Fizazi et al. 2011; Henry et al. 2011]. The incidence of ONJ was low in both treatment arms (2% with denosumab versus 1% with ZOL, p = 0.09, in the CRPC trial; 1.1% with denosumab versus 1.3% with ZOL, p = 1.00, in the trial in patients with multiple myeloma or bone metastases from solid tumors other than breast and prostate cancers) [Fizazi et al. 2011; Henry et al. 2011]. Compared with ZOL, denosumab was associated with fewer acute-phase reactions (approximately half the rate compared with ZOL) and increased incidence of hypocalcemia (~2× higher rates than with ZOL) in these trials [Fizazi et al. 2011; Henry et al. 2011]. The key safety data from these trials are summarized in Table 3 [Fizazi et al. 2011; Henry et al. 2011]. Similar to the efficacy outcomes with denosumab, detailed safety data in the subsets of patients with RCC or bladder cancer are yet to be reported [Fizazi et al. 2011; Henry et al. 2011].
Table 3.
Efficacy and safety data from the phase III trials comparing denosumab versus zoledronic acid in patients with genitourinary malignancies.
| Endpoint | CRPC [Fizazi et al. 2011; Henry et al. 2011] | Multiple myeloma or solid tumors (including RCC and bladder cancer) [Fizazi et al. 2011; Henry et al. 2011] |
|---|---|---|
| Efficacy | ||
| Median time to first SRE | 20.7 months (denosumab) versus 17.1 months (ZOL); p=0.008 for superiority | 20.6 months (denosumab) versus 16.3 months (ZOL); p=0.06 for superiority |
| Cumulative risk of SREs | HR = 0.82; p=0.008 for denosumab versus ZOL | HR = 0.90; p=0.14 for denosumab versus ZOL |
| Safety* | ||
| Acute-phase reactions | 8% (denosumab) versus 18% (ZOL); p not reported | 6.9% (denosumab) versus 14.5% (ZOL); p<0.001 |
| Hypocalcemia | 13% (denosumab) versus 6% (ZOL); p<0.0001 | 10.8% (denosumab) versus 5.8% (ZOL); p not reported |
| Renal adverse events | 15% (denosumab) versus 16% (ZOL); p not reported | 8.3% (denosumab) versus 10.9% (ZOL): p=0.07 |
| Osteonecrosis of the jaw | 2% (denosumab) versus 1% (ZOL); p=0.09 | 1.3% (denosumab) versus 1.1% (ZOL); p=1.00 |
Only AEs of interest based on known safety profiles of the two agents are presented.
Abbreviations: CRPC, castration-resistant prostate cancer; HR, hazard ratio; RCC, renal cell carcinoma.
The phase III trials comparing denosumab with ZOL in patients with bone metastases excluded patients with severe renal impairment (per the ZOL prescribing information); therefore, the safety of denosumab in patients with severe renal impairment is unknown. However, earlier phase II studies with different doses of denosumab indicated increased potential for severe hypocalcemia in patients with renal impairment [Amgen Inc., 2010], suggesting that additional trials are necessary before denosumab can be recommended in patients with severe renal impairment. Indeed, current prescribing information for denosumab highlights this potential issue [Amgen Inc., 2010], and recommends monitoring serum calcium levels in addition to calcium and vitamin D supplementation in patients receiving denosumab therapy. However, the prescribing information for denosumab does not specify the frequency or timing of serum calcium monitoring relative to administration of the antiresorptive. In the absence of clearly defined monitoring protocols for these patients, the onus is on the treating physician to devise and implement such monitoring protocols to ensure patients’ safety [Aapro and Launay-Vacher, 2011].
Antiresorptive therapy: a transient intervention or long-term treatment?
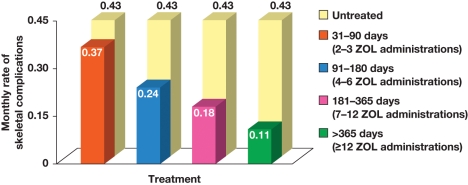
Phase III trials of antiresorptive agents have typically treated patients for 2 years or less on study [Saad et al. 2004], resulting in a paucity of long-term efficacy and safety data. As treatment options in GU malignancies evolve, OS continues to improve, even for patients with bone metastases. As a result, physicians are faced with the choice of stopping bone-directed therapy after a finite length of time, or continuously treating their patients for as long as the therapy is tolerated. Current treatment guidelines provide little guidance on this aspect [British Association of Urological Surgeons, 2005; Heidenreich et al. 2009]. However, retrospective database analyses have demonstrated increasing SRE- and fracture-reduction benefits with longer treatment persistency. For example, in a mixed population of patients with bone metastases from solid tumors, ZOL treatment for any duration reduced the incidence of SREs compared with no bone-modifying therapy; however, persistence with ZOL for longer than 12 months was associated with a substantially greater reduction in SRE rate compared with ZOL treatment for 1–3 months (Figure 4) [Hatoum et al. 2008].
Figure 4.
Persistency with zoledronic acid (ZOL) treatment was associated with a lower monthly rate of skeletal-related events in patients with bone metastases from solid tumors. Reproduced with permission from Hatoum et al. [2008]. © 2008 American Cancer Society.
A second database analysis evaluated patients with bone metastases from PC who received ZOL ‘early’ (i.e. before their first SRE) or ‘late’ (i.e. after one or more SREs had occurred) [Hatoum et al. 2011]. In this analysis, late initiation of ZOL after SRE incidence was associated with a 1.5-fold increased risk of a subsequent SRE compared with early ZOL treatment [Hatoum et al. 2011]. Furthermore, longer persistency with ZOL was associated with increasing benefits in this population [Hatoum et al. 2011], similar to the effects observed in the larger population of patients with bone metastases from solid tumors [Hatoum et al. 2008].
Antiresorptive agents and benefits beyond bone health
Preclinical and translational data suggest potential anticancer activity of bisphosphonates
Bone-modifying agents can alter the bone microenvironment and may thereby disrupt interactions between PC cells and bone that are central to metastatic tumor formation [Josson et al. 2010; Mundy, 2002; Rucci and Teti, 2010]. In a preclinical model, orchiectomy was associated with elevated rates of bone loss and PC metastasis to bone [Padalecki et al. 2002], both of which were reversed by ZOL treatment [Padalecki et al. 2002]. Bisphosphonates may also inhibit disease progression by stimulating innate antitumor immune mechanisms such as γδ T cells, which are activated by phosphoantigens and signaling intermediates often overexpressed in cancer cells [Clezardin and Massaia, 2010]. Nitrogen-containing BPs such as ZOL block the mevalonate biosynthesis pathway in target cells, resulting in phosphoantigen accumulation and associated effects on γδ T-cell activity [Clezardin and Massaia, 2010]. For example, in patients with PC, ZOL treatment elicited a long-term shift of peripheral γδ cells toward an activated effector memory-like state associated with improved immune surveillance against transformed or malignant cells [Dieli et al. 2007; Naoe et al. 2010]. In addition, preclinical studies suggest that BPs may also have direct anticancer activity (e.g. induction of cancer cell apoptosis) and synergy with cytotoxic chemotherapy, and effects are especially profound for ZOL [Boissier et al. 2000; Clyburn et al. 2010; Coxon et al. 2004; Facchini et al. 2010; Koul et al. 2010; Morgan et al. 2007; Neville-Webbe et al. 2005].
Preclinical studies in models of other GU cancers have also shown that BPs (especially ZOL) can inhibit overall tumor progression, proliferation, invasion, and angiogenesis; activate immune response against cancer cells; promote apoptosis; and produce synergistic anticancer effects with cytotoxic agents [Guise, 2008; Soltau et al. 2008; Ullen et al. 2009; Yuasa et al. 2009]. The observed antiangiogenic effect of ZOL is especially intriguing given the extensive vascularization characteristic of RCC and the success of antiangiogenic therapies to treat metastatic RCC [Choueiri et al. 2010], and merits further investigation.
Clinical evidence for using antiresorptive agents to delay disease progression
Early trials of clodronate, an oral BP, in patients with hormone-sensitive PC yielded provocative data suggesting an effect on the disease course. Two studies evaluated the effect of clodronate on skeletal health and OS in men receiving hormonal therapy for stage M0 (localized disease; N = 508) or M1 (bone metastases; N = 311) PC [Dearnaley et al. 2009a]. In long-term follow up, clodronate significantly improved OS in men with M1 disease beginning hormonal therapy (HR = 0.77; p = 0.032), but not in men with M0 disease (HR = 1.12; p = 0.94) [Dearnaley et al. 2009a]. Moreover, in the phase III placebo-controlled trial of ZOL for reducing the risk of SREs in men with bone metastases from CRPC, ZOL produced a trend toward improved OS (median survival 546 days versus 469 with placebo; p = 0.103) [Saad, 2008].
Similar to the data in CRPC, retrospective analyses of the RCC subset from the phase III trial of ZOL in patients with lung cancer or other solid tumors showed that ZOL significantly extended time to disease progression (586 versus 89 days; p = 0.014) [Saad and Lipton, 2005] and demonstrated a trend toward prolonged OS (347 versus 216 days; p = 0.104) compared with placebo [Lipton et al. 2004; Saad, 2008; Saad and Lipton, 2005]. Moreover, in a prospective, placebo-controlled trial in patients with bone metastases from bladder cancer (N = 40), ZOL (4 mg intravenously monthly for 6 months) significantly increased the 1-year survival rate (36.3 ± 11.2% versus 0% for placebo; p = 0.004; median survival not reported) compared with placebo [Zaghloul et al. 2010], further supporting potential beneficial effects of ZOL on disease progression in advanced GU malignancies.
Exploratory analyses from phase III placebo-controlled trials of zoledronic acid suggest additional benefits in patients with elevated levels of bone turnover markers
Biochemical markers of bone turnover reflect the ongoing dynamics of bone remodeling, and include peptides (e.g. N-telopeptide of type I collagen [NTX]) and enzymes (e.g. bone-specific alkaline phosphatase) that are highly specific to bone. These markers are released during bone remodeling into serum and secreted in urine, and can thereby be assessed using noninvasive methodology. In retrospective exploratory analyses of the databases from phase III trials comparing ZOL versus placebo in patients with bone metastases from CRPC or other solid tumors, elevated bone marker levels (at baseline or during BP treatment) were associated with increased risk of SREs and reduced survival [Brown et al. 2005; Coleman et al. 2005; Cook et al. 2006; Smith et al. 2007]. In addition, pilot trials suggest potential associations between elevated bone marker levels and increased risks of disease recurrence or progression [Costa et al. 2009; Noguchi et al. 2003], supporting the hypothesis that normalization of elevated bone turnover might correlate with better disease outcomes. Indeed, in exploratory analyses of data from the phase III trial of ZOL versus placebo in patients with bone metastases from CRPC (N = 314), 70% of ZOL-treated patients who had elevated baseline NTX (≥64 nmol/mmol creatinine; n = 193) normalized their NTX levels within 3 months, compared with only 8% in the placebo group. In these analyses, normalization of NTX levels was associated with 59% decrease in the risk of death (p < 0.0001) compared with persistently elevated NTX levels [Lipton et al. 2008], and any decrease in NTX over the first 3 months was associated with a corresponding improvement in survival [Lipton et al. 2008]. Interestingly, further retrospective analyses of the phase III trial database of ZOL showed that patients with aggressive bone disease (defined by markedly elevated NTX levels ≥100 nmol/mmol creatinine) at baseline elicited an OS benefit from ZOL treatment [Body et al. 2009]. Interestingly, although SREs (especially pathologic fractures) are associated with increased mortality [Saad et al. 2007], the OS benefit with ZOL in this patient subset was maintained in analyses adjusting for SRE incidence [Body et al. 2009], thereby suggesting a true effect on the course of the underlying disease.
Exploratory analyses in patients with bone metastases from RCC showed very similar results to the CRPC analyses described above. Patients with bone metastases from RCC and elevated (≥64 nmol/mmol creatinine) NTX on study had an increased risk of death (HR = 13.370; p = 0.0001), bone disease progression (HR = 11.137; p = 0.0087), first pathologic fracture (HR = 7.650; p = 0.0217), and any pathologic fracture (HR = 5.085; p = 0.0031) compared with patients with normal baseline NTX levels [Brown et al. 2009]. Normalization of elevated baseline NTX levels within 3 months of ZOL therapy was also associated with a reduced risk of death compared with persistently elevated NTX in patients with solid tumors (including RCC and bladder cancer) [Lipton et al. 2008].
To date, the placebo-controlled trials of ZOL have provided a wealth of correlative data exploring the relationship between bone turnover levels and disease outcomes. The recent head-to-head trial comparing denosumab versus ZOL has also collected bone marker data prospectively [Fizazi et al. 2011; Henry et al. 2011]. Further analyses of those data will provide insights into the general applicability of the correlations observed in ZOL-treated patients.
Ongoing trials are evaluating the potential for BPs and other bone-modifying agents to delay or prevent prostate cancer progression (Table 4) [Dearnaley et al. 2009b; Smith et al. 2012; US National Institutes of Health, 2009, 2011b]. Results from the trials evaluating ZOL are awaited, and the phase III trial evaluating the role of denosumab in prolonging time to bone metastases in patients with CRPC (N = 1432) has recently reported improvement in bone-metastasis-free survival by 4.2 months (HR = 0.85; p = 0.028) versus placebo [Smith et al. 2012]. This delay in the development of bone metastases was, however, not accompanied by significant improvements in overall progression-free survival (HR=0.89; p=0.09) or OS (HR = 1.01; p = 0.91) [Smith et al. 2012], suggesting that modulating the bone microenvironment might not, in itself, be sufficient to delay PC progression. Denosumab appeared to be well tolerated overall, although increased rates of ONJ (5% overall in the denosumab arm versus none in the placebo arm) [Smith et al. 2012] are a substantial concern in this group of patients, few of whom had received prior chemotherapy. Additional details from this trial are awaited, including extraskeletal disease progression and patient exposure to denosumab (the trial was initiated with 60 mg denosumab dosed every 4 weeks, and later increased to 120 mg every 4 weeks) [Smith et al. 2012; US National Institutes of Health, 2011a]. Nonetheless, these promising results from the denosumab trial have increased interest in outcomes from ongoing trials of antiresorptive agents such as ZOL (which may combine anticancer effects on PC cells with its ability to modify the bone microenvironment).
Table 4.
Ongoing phase III trials of antiresorptive agents to delay disease progression in the prostate cancer setting.
| Study (agent) | N | Accrual status | Key endpoints | Results |
|---|---|---|---|---|
| ZEUS (ZOL) | 1498 | Complete | Time to bone metastasis in high-risk M0 disease (± ADT) | Awaited |
| RADAR (ZOL) | 1071 | Complete | Relapse-free survival in patients receiving short- or long-term ADT | Awaited |
| STAMPEDE (ZOL) | 3300 | Enrolling; currently n=1469 | Failure-free survival in patients receiving ADT ± chemotherapy | Awaited |
| AMG147 (denosumab) | 1432 | Complete | Time to bone metastasis or death in high-risk, nonmetastatic CRPC | ↑ BMFS versus placebo; no effect on OS or overall disease progression [Smith et al. 2012] |
Abbreviations: ADT, androgen-deprivation therapy; BMFS, bone-metastasis-free survival; CRPC, castration-resistant prostate cancer; OS, overall survival; ZOL, zoledronic acid.
Conclusions
Preserving bone health is an important consideration in the management of patients with advanced GU malignancies. This need is further highlighted in PC, wherein anticancer treatments and patient age can further exacerbate challenges to skeletal health. For nearly a decade, ZOL has been the mainstay of treatment to reduce the risk of SREs in patients with bone metastases from GU cancers, and has shown promise for preserving BMD during anticancer therapy. A wealth of clinical experience underscores the practical benefits and tolerability of ZOL in advanced oncology. Moreover, clinical studies suggest a possible beneficial effect of ZOL on disease progression, which awaits confirmation in ongoing trials. Recently, the approval of denosumab for the treatment of bone metastases from solid tumors has provided an additional option for antiresorptive therapy in advanced GU cancers, and newly approved anticancer agents may also exert beneficial effects on bone. Overall, the division between anticancer and bone-directed (supportive care) agents is now blurring, and ongoing studies are expected to further expand the therapeutic repertoire in GU cancers.
Acknowledgments
Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals. We thank Shalini Murthy, PhD, ProEd Communications, Inc., for her medical editorial assistance with this manuscript.
Footnotes
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. Medical editorial assistance was funded by Novartis.
Dr Aapro has conducted studies and is a consultant on bone-modifying agents for Amgen, Bayer-Schering, Novartis, and Roche. Dr Saad has served as an advisor and conducted research for Novartis and Amgen.
References
- Aapro M., Abrahamsson P.A., Body J.J., Coleman R.E., Colomer R., Costa L., et al. (2008) Guidance on the use of bisphosphonates in solid tumours: recommendations of an international expert panel. Ann Oncol 19: 420–432 [DOI] [PubMed] [Google Scholar]
- Aapro M., Launay-Vacher V. (2011) Importance of monitoring renal function in patients with cancer. Cancer Treat Rev [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Abrahamsson P.A., Ostri P., Andersen M., Kruger Hagen E. (2008) Nordic observational study evaluating safety and analgesic consumption in patients with advanced cancer under zoledronic acid (ZOMETA®) treatment: NOSAZ—interim analysis. Poster session presented at: 23rd Annual EAU Congress, 26–29 March 2008, Milan, Italy Abstract 645 [Google Scholar]
- Amgen Europe B.V (2010) Prolia [summary of product characteristics]. Amgen Europe B.V.: Breda, The Netherlands [Google Scholar]
- Amgen Inc (2010) Xgeva (denosumab) injection [package insert]. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/125320s007lbl.pdf Amgen Inc.: Thousand Oaks, CA [Google Scholar]
- Amgen Inc (2011) Xgeva (denosumab) granted marketing authorization in the European Union [press release]. Available at: http://www.amgen.com/media/media_pr_detail.jsp?releaseID=1585714 Amgen Inc.: Thousand Oaks, CA [Google Scholar]
- Anand A., Scher H.I., Beer T.M., Higano C.S., Danila D.C., Taplin M., et al. (2010) Circulating tumor cells (CTC) and prostate specific antigen (PSA) as response indicator biomarkers in chemotherapy-naive patients with progressive castration-resistant prostate cancer (CRPC) treated with MDV3100. J Clin Oncol 28(15 Suppl.): 353s Abstract 4546 [Google Scholar]
- Aus G., Abbou C.C., Bolla M., Heidenreich A., Schmid H.P., van Poppel H., et al. (2005) EAU guidelines on prostate cancer. Eur Urol 48: 546–551 [DOI] [PubMed] [Google Scholar]
- Babjuk M., Oosterlinck W., Sylvester R., Kaasinen E., Bohle A., Palou-Redorta J., et al. (2011) EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder, the 2011 update. Eur Urol 59: 997–1008 [DOI] [PubMed] [Google Scholar]
- Berg A., Berner A., Lilleby W., Bruland O.S., Fossa S.D., Nesland J.M., et al. (2007) Impact of disseminated tumor cells in bone marrow at diagnosis in patients with nonmetastatic prostate cancer treated by definitive radiotherapy. Int J Cancer 120: 1603–1609 [DOI] [PubMed] [Google Scholar]
- Bhoopalam N., Campbell S.C., Moritz T., Broderick W.R., Iyer P., Arcenas A.G., et al. (2009) Intravenous zoledronic acid to prevent osteoporosis in a veteran population with multiple risk factors for bone loss on androgen deprivation therapy. J Urol 182: 2257–2264 [DOI] [PubMed] [Google Scholar]
- Body J.-J., Cook R., Costa L., Brown J.E., Terpos E., Saad F., et al. (2009) Possible survival benefits from zoledronic acid treatment in patients with bone metastases from solid tumors and poor prognostic features. Poster session presented at: The IX International Meeting on Cancer Induced Bone Disease, 28–31 October 2009, Arlington, VA Poster 71 [Google Scholar]
- Boissier S., Ferreras M., Peyruchaud O., Magnetto S., Ebetino F.H., Colombel M., et al. (2000) Bisphosphonates inhibit breast and prostate carcinoma cell invasion, an early event in the formation of bone metastases. Cancer Res 60: 2949–2954 [PubMed] [Google Scholar]
- Boyle W.J., Simonet W.S., Lacey D.L. (2003) Osteoclast differentiation and activation. Nature 423: 337–342 [DOI] [PubMed] [Google Scholar]
- British Association of Urological Surgeons (2005) Metastatic Prostate Cancer Guidelines. London, UK: The British Association of Urological Surgeons; http://www.echurology.co.uk/baus.html [Google Scholar]
- Brown J.E., Cook R.J., Major P., Lipton A., Saad F., Smith M., et al. (2005) Bone turnover markers as predictors of skeletal complications in prostate cancer, lung cancer, and other solid tumors. J Natl Cancer Inst 97: 59–69 [DOI] [PubMed] [Google Scholar]
- Brown J.E., Lipton A., Cook R.J., Michaelson M.D., Saad F. (2009) N-telopeptide of type I collagen (NTX) correlates with survival and fractures in patients (pts) with bone metastases from renal cell carcinoma (RCC). Poster session presented at: American Society of Clinical Oncology 2009 Genitourinary Cancers Symposium, 26–28 February 2009, Orlando, FL Poster A66 [Google Scholar]
- Casey R., Gesztesi Z., Rochford J. (2010) Long term zoledronic acid during androgen blockade for prostate cancer. Can J Urol 17: 5170–5177 [PubMed] [Google Scholar]
- Choueiri T.K., Duh M.S., Clement J., Brick A.J., Rogers M.J., Kwabi C., et al. (2010) Angiogenesis inhibitor therapies for metastatic renal cell carcinoma: effectiveness, safety and treatment patterns in clinical practice-based on medical chart review. BJU Int 105: 1247–1254 [DOI] [PubMed] [Google Scholar]
- Clezardin P., Massaia M. (2010) Nitrogen-containing bisphosphonates and cancer immunotherapy. Curr Pharm Des 16: 3007–3014 [DOI] [PubMed] [Google Scholar]
- Clines G.A., Guise T.A. (2008) Molecular mechanisms and treatment of bone metastasis. Expert Rev Mol Med 10: e7. [DOI] [PubMed] [Google Scholar]
- Clyburn R.D., Reid P., Evans C.A., Lefley D.V., Holen I. (2010) Increased anti-tumour effects of doxorubicin and zoledronic acid in prostate cancer cells in vitro: supporting the benefits of combination therapy. Cancer Chemother Pharmacol 65: 969–978 [DOI] [PubMed] [Google Scholar]
- Coleman R.E. (2001) Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev 27: 165–176 [DOI] [PubMed] [Google Scholar]
- Coleman R.E., Major P., Lipton A., Brown J.E., Lee K.A., Smith M., et al. (2005) Predictive value of bone resorption and formation markers in cancer patients with bone metastases receiving the bisphosphonate zoledronic acid. J Clin Oncol 23: 4925–4935 [DOI] [PubMed] [Google Scholar]
- Cook R.J., Coleman R., Brown J., Lipton A., Major P., Hei Y.J., et al. (2006) Markers of bone metabolism and survival in men with hormone-refractory metastatic prostate cancer. Clin Cancer Res 12: 3361–3367 [DOI] [PubMed] [Google Scholar]
- Costa L., Cook R., Body J.-J., Brown J.E., Terpos E., Saad F., et al. (2009) Zoledronic acid treatment delays disease progression and improves survival in patients with bone metastases from solid tumors and elevated levels of bone resorption. Poster session presented at: The IX International Meeting on Cancer Induced Bone Disease, 28–31 October 2009, Arlington, VA Poster 50 [Google Scholar]
- Coxon J.P., Oades G.M., Kirby R.S., Colston K.W. (2004) Zoledronic acid induces apoptosis and inhibits adhesion to mineralized matrix in prostate cancer cells via inhibition of protein prenylation. BJU Int 94: 164–170 [DOI] [PubMed] [Google Scholar]
- Daniell H.W., Dunn S.R., Ferguson D.W., Lomas G., Niazi Z., Stratte P.T. (2000) Progressive osteoporosis during androgen deprivation therapy for prostate cancer. J Urol 163: 181–186 [PubMed] [Google Scholar]
- Danila D.C., Anand A., Sung C.C., Leversha M., Rathkopf D.E., Morris M.J., et al. (2010) Molecular profiling of circulating tumor cells (CTC) in patients with castrate metastatic prostate cancer (CMPC) receiving abiraterone acetate (AA) after failure of docetaxel-based chemotherapy. J Clin Oncol 28(15 Suppl.): 375s Abstract 4635 [Google Scholar]
- Danila D.C., Heller G., Gignac G.A., Gonzalez-Espinoza R., Anand A., Tanaka E., et al. (2007) Circulating tumor cell number and prognosis in progressive castration-resistant prostate cancer. Clin Cancer Res 13: 7053–7058 [DOI] [PubMed] [Google Scholar]
- de Bono J.S., Logothetis C.J., Molina A., Fizazi K., North S., Chu L., et al. (2011) Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 364: 1995–2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearnaley D.P., Mason M.D., Parmar M.K., Sanders K., Sydes M.R. (2009a) Adjuvant therapy with oral sodium clodronate in locally advanced and metastatic prostate cancer: long-term overall survival results from the MRC PR04 and PR05 randomised controlled trials. Lancet Oncol 10: 872–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearnaley D.P., Mason M.D., Parmar M.K., Sanders K., Sydes M.R. (2009b) Survival benefit with oral sodium clodronate in metastatic but not localised prostate cancer: long-term results of MRC PR04 & PR05. Oral session presented at: American Society of Clinical Oncology 2009 Genitourinary Cancers Symposium, 26–28 February 2009, Orlando, FL Abstract 6 [Google Scholar]
- Dieli F., Vermijlen D., Fulfaro F., Caccamo N., Meraviglia S., Cicero G., et al. (2007) Targeting human γδ T cells with zoledronate and interleukin-2 for immunotherapy of hormone-refractory prostate cancer. Cancer Res 67: 7450–7457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donat D.A., Pesic J.M., Tesanovic S.M., Donat D.D. (2006) Low-dose clodronate as adjunctive therapy in prostate cancer patients with painful bone metastases. Ann Oncol 17(Suppl. 9): ix156 Abstract 484 [Google Scholar]
- Ernst D.S., Tannock I.F., Winquist E.W., Venner P.M., Reyno L., Moore M.J., et al. (2003) Randomized, double-blind, controlled trial of mitoxantrone/prednisone and clodronate versus mitoxantrone/prednisone and placebo in patients with hormone-refractory prostate cancer and pain. J Clin Oncol 21: 3335–3342 [DOI] [PubMed] [Google Scholar]
- Facchini G., Caraglia M., Morabito A., Marra M., Piccirillo M.C., Bochicchio A.M., et al. (2010) Metronomic administration of zoledronic acid and Taxotere combination in castration resistant prostate cancer patients: phase I ZANTE trial. Cancer Biol Ther 10: 543–548 [DOI] [PubMed] [Google Scholar]
- Fizazi K., Carducci M., Smith M., Damiao R., Brown J., Karsh L., et al. (2011) Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet 377: 813–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia M., Jemal A., Ward E.M., Center M., Hao Y., Siegel R.L., et al. (2007) Global cancer facts and figures 2007. Available at: http://www.cancer.org/acs/groups/content/@nho/documents/document/globalfactsandfigures2007rev2p.pdf Atlanta, GA: American Cancer Society [Google Scholar]
- Greenspan S.L., Bone H.G., Ettinger M.P., Hanley D.A., Lindsay R., Zanchetta J.R., et al. (2007) Effect of recombinant human parathyroid hormone (1-84) on vertebral fracture and bone mineral density in postmenopausal women with osteoporosis: a randomized trial. Ann Intern Med 146: 326–339 [DOI] [PubMed] [Google Scholar]
- Guise T.A. (2008) Antitumor effects of bisphosphonates: promising preclinical evidence. Cancer Treat Rev 34(Suppl. 1): S19–S24 [DOI] [PubMed] [Google Scholar]
- Hatoum H.T., Lin S.J., Guo A., Lipton A., Smith M.R. (2011) Zoledronic acid therapy impacts risk and frequency of skeletal complications and follow-up duration in prostate cancer patients with bone metastasis. Curr Med Res Opin 27: 55–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatoum H.T., Lin S.J., Smith M.R., Barghout V., Lipton A. (2008) Zoledronic acid and skeletal complications in patients with solid tumors and bone metastases: analysis of a national medical claims database. Cancer 113: 1438–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidenreich A., Bolla M., Joniau S., van der Kwast T.H., Matveev V., Mason M.D., et al. (2009) Guidelines on prostate cancer. Available at: http://www.uroweb.org/fileadmin/tx_eauguidelines/2009/Full/Prostate_Cancer.pdf Arnhem, The Netherlands: European Association of Urology [Google Scholar]
- Heidenreich A., Ohlmann C., Engelmann U.H. (2005) Ibandronate in the management of painful osseous metastases due to hormone refractory prostate cancer. Poster session presented at: 2005 American Society of Clinical Oncology Prostate Cancer Symposium, 17–19 February 2005, Orlando, FL [Google Scholar]
- Henry D.H., Costa L., Goldwasser F., Hirsh V., Hungria V., Prausova J., et al. (2011) Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol 29: 1125–1132 [DOI] [PubMed] [Google Scholar]
- Hering F., Rodrigues P.R., Lipay M. (2003) Clodronate for treatment of bone metastases in hormone refractory prostate cancer. Int Braz J Urol 29: 228–233 [DOI] [PubMed] [Google Scholar]
- Hird A.E., Chow E., Ehrlich L., Probyn L., Sinclair E., Yip D., et al. (2008) Rapid improvement in pain and functional level in a patient with metastatic renal cell carcinoma: a case report and review of the literature. J Palliat Med 11: 1156–1161 [DOI] [PubMed] [Google Scholar]
- Hoff A.O., Toth B.B., Altundag K., Johnson M.M., Warneke C.L., Hu M., et al. (2008) Frequency and risk factors associated with osteonecrosis of the jaw in cancer patients treated with intravenous bisphosphonates. J Bone Miner Res 23: 826–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israeli R.S., Rosenberg S.J., Saltzstein D.R., Gottesman J.E., Goldstein H.R., Hull G.W., et al. (2007) The effect of zoledronic acid on bone mineral density in patients undergoing androgen deprivation therapy. Clin Genitourin Cancer 5: 271–277 [DOI] [PubMed] [Google Scholar]
- Izumi K., Mizokami A., Sugimoto K., Narimoto K., Miwa S., Maeda Y., et al. (2009) Risedronate recovers bone loss in patients with prostate cancer undergoing androgen-deprivation therapy. Urology 73: 1342–1346 [DOI] [PubMed] [Google Scholar]
- Josson S., Matsuoka Y., Chung L.W., Zhau H.E., Wang R. (2010) Tumor-stroma co-evolution in prostate cancer progression and metastasis. Semin Cell Dev Biol 21: 26–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kijima T., Fujii Y., Suyama T., Okubo Y., Yamamoto S., Masuda H., et al. (2009) Radiotherapy to bone metastases from renal cell carcinoma with or without zoledronate. BJU Int 103: 620–624 [DOI] [PubMed] [Google Scholar]
- Kollermann J., Weikert S., Schostak M., Kempkensteffen C., Kleinschmidt K., Rau T., et al. (2008) Prognostic significance of disseminated tumor cells in the bone marrow of prostate cancer patients treated with neoadjuvant hormone treatment. J Clin Oncol 26: 4928–4933 [DOI] [PubMed] [Google Scholar]
- Koul H.K., Koul S., Kumar B., Meacham R.B., Maroni P. (2010) Direct effects of zoledronic acid on hormone-responsive and hormone-refractory prostate cancer cells. Poster session presented at: American Society of Clinical Oncology 2010 Genitourinary Cancers Symposium, 5–7 March 2010, San Francisco, CA Abstract 184 [Google Scholar]
- Launay-Vacher V., Ayllon J., Janus N., Spano J.P., Ray-Coquard I., Gligorov J., et al. (2009a) Drug management of prostate cancer: prevalence and consequences of renal insufficiency. Clin Genitourin Cancer 7(3): E83–E89 [DOI] [PubMed] [Google Scholar]
- Launay-Vacher V., Oudard S., Janus N., Gligorov J., Pourrat X., Rixe O., et al. (2007) Prevalence of renal insufficiency in cancer patients and implications for anticancer drug management: the Renal Insufficiency and Anticancer Medications (IRMA) study. Cancer 110: 1376–1384 [DOI] [PubMed] [Google Scholar]
- Launay-Vacher V., Spano J.P., Janus N., Gligorov J., Ray-Coquard I., Oudard S., et al. (2009b) Renal insufficiency and anticancer drugs in elderly cancer patients: a subgroup analysis of the IRMA study. Crit Rev Oncol Hematol 70: 124–133 [DOI] [PubMed] [Google Scholar]
- Lee R.J., Stott S.L., Nagrath S., Ulkus L.E., Dahl D.M., Smith M.R., et al. (2009) Analyses of circulating tumor cell (CTC) dynamics and treatment response in prostate cancer using CTC-chip microfluidic device. J Clin Oncol 27(15 Suppl.): 271s Abstract 5149 [Google Scholar]
- Lipton A., Cook R., Saad F., Major P., Garnero P., Terpos E., et al. (2008) Normalization of bone markers is associated with improved survival in patients with bone metastases from solid tumors and elevated bone resorption receiving zoledronic acid. Cancer 113: 193–201 [DOI] [PubMed] [Google Scholar]
- Lipton A., Seaman J., Zheng M. (2004) Efficacy and safety of zoledronic acid in patients with bone metastases from renal cell carcinoma. Poster session presented at: What Is New in Bisphosphonates? Seventh Workshop on Bisphosphonates—From the Laboratory to the Patient, 24–26 March 2004, Davos, Switzerland Abstract 28 [Google Scholar]
- Lipton A., Small E., Saad F., Gleason D., Gordon D., Smith M., et al. (2002) The new bisphosphonate, Zometa (zoledronic acid), decreases skeletal complications in both osteolytic and osteoblastic lesions: a comparison to pamidronate. Cancer Invest 20(Suppl. 2): 45–54 [DOI] [PubMed] [Google Scholar]
- Lipton A., Zheng M., Seaman J. (2003) Zoledronic acid delays the onset of skeletal-related events and progression of skeletal disease in patients with advanced renal cell carcinoma. Cancer 98: 962–969 [DOI] [PubMed] [Google Scholar]
- Logothetis C., De Bono J.S., Molina A., Basch E.M., Fizazi K., North S.A., et al. (2011) Effect of abiraterone acetate (AA) on pain control and skeletal-related events (SRE) in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) post docetaxel (D): results from the COU-AA-301 phase III study. J Clin Oncol 29(Suppl.): abstract 4520 [Google Scholar]
- Meads M.B., Hazlehurst L.A., Dalton W.S. (2008) The bone marrow microenvironment as a tumor sanctuary and contributor to drug resistance. Clin Cancer Res 14: 2519–2526 [DOI] [PubMed] [Google Scholar]
- Mittan D., Lee S., Miller E., Perez R.C., Basler J.W., Bruder J.M. (2002) Bone loss following hypogonadism in men with prostate cancer treated with GnRH analogs. J Clin Endocrinol Metab 87: 3656–3661 [DOI] [PubMed] [Google Scholar]
- Miwa S., Mizokami A., Konaka H., Izumi K., Nohara T., Namiki M. (2009) A case of bone, lung, pleural and liver metastases from renal cell carcinoma which responded remarkably well to zoledronic acid monotherapy. Jpn J Clin Oncol 39: 745-750 [DOI] [PubMed] [Google Scholar]
- Molina A.M., Motzer R.J. (2011) Clinical practice guidelines for the treatment of metastatic renal cell carcinoma: today and tomorrow. Oncologist 16(Suppl. 2): 45–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan C., Lewis P.D., Jones R.M., Bertelli G., Thomas G.A., Leonard R.C. (2007) The in vitro anti-tumour activity of zoledronic acid and docetaxel at clinically achievable concentrations in prostate cancer. Acta Oncol 46: 669–677 [DOI] [PubMed] [Google Scholar]
- Morgan T.M., Lange P.H., Porter M.P., Lin D.W., Ellis W.J., Gallaher I.S., et al. (2009) Disseminated tumor cells in prostate cancer patients after radical prostatectomy and without evidence of disease predicts biochemical recurrence. Clin Cancer Res 15: 677–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulders P.F., Miller K., Tchekmedyian N.S., Chen Y.M. (2007) Long-term reduction in risk of skeletal complications with zoledronic acid in patients with advanced renal cell carcinoma or bladder cancer. Oral session presented at: 22nd Annual EAU Congress, 21–24 March 2007, Berlin, Germany Abstract 971 [Google Scholar]
- Mundy G.R. (2002) Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer 2: 584–593 [DOI] [PubMed] [Google Scholar]
- Naoe M., Ogawa Y., Takeshita K., Morita J., Shichijo T., Fuji K., et al. (2010) Zoledronate stimulates gamma delta T cells in prostate cancer patients. Oncol Res 18: 493–501 [DOI] [PubMed] [Google Scholar]
- National Comprehensive Cancer Network (2009) NCCN clinical practice guidelines in oncology: prostate cancer, v.2.2009. Available at: http://www.nccn.org Fort Washington, PA: National Comprehensive Cancer Network, Inc; [DOI] [PubMed] [Google Scholar]
- Neville-Webbe H.L., Rostami-Hodjegan A., Evans C.A., Coleman R.E., Holen I. (2005) Sequence- and schedule-dependent enhancement of zoledronic acid induced apoptosis by doxorubicin in breast and prostate cancer cells. Int J Cancer 113: 364–371 [DOI] [PubMed] [Google Scholar]
- Noguchi M., Yahara J., Noda S. (2003) Serum levels of bone turnover markers parallel the results of bone scintigraphy in monitoring bone activity of prostate cancer. Urology 61: 993–998 [DOI] [PubMed] [Google Scholar]
- Norton L., Massague J. (2006) Is cancer a disease of self-seeding? Nat Med 12: 875–878 [DOI] [PubMed] [Google Scholar]
- Novartis Pharmaceuticals Corporation (2011) Zometa® (zoledronic acid) injection [package insert]. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/021386s004lbl.pdf East Hanover, NJ: Novartis Pharmaceuticals Corporation [Google Scholar]
- Olmos D., Arkenau H.T., Ang J.E., Ledaki I., Attard G., Carden C.P., et al. (2009) Circulating tumour cell (CTC) counts as intermediate end points in castration-resistant prostate cancer (CRPC): a single-centre experience. Ann Oncol 20: 27–33 [DOI] [PubMed] [Google Scholar]
- Padalecki S.S., Carreon M.R., Grubbs B., Cui Y., Guise T.A. (2002) Androgen deprivation causes bone loss and increased prostate cancer metastases to bone: prevention by zoledronic acid. Poster session presented at: 24th Annual Meeting of the American Society for Bone and Mineral Research, 20–24 September 2002, San Antonio, TX Abstract SU072 [Google Scholar]
- Paget S. (1889) The distribution of secondary growths in cancer of the breast. Lancet 1: 571–573 [PubMed] [Google Scholar]
- Parker C.C. (2005) The role of bisphosphonates in the treatment of prostate cancer. BJU Int 95: 935–938 [DOI] [PubMed] [Google Scholar]
- Planas J., Trilla E., Raventos C., Cecchini L., Orsola A., Salvador C., et al. (2009) Alendronate decreases the fracture risk in patients with prostate cancer on androgen-deprivation therapy and with severe osteopenia or osteoporosis. BJU Int 104: 1637–1640 [DOI] [PubMed] [Google Scholar]
- Preston D.M., Torrens J.I., Harding P., Howard R.S., Duncan W.E., McLeod D.G. (2002) Androgen deprivation in men with prostate cancer is associated with an increased rate of bone loss. Prostate Cancer Prostatic Dis 5: 304–310 [DOI] [PubMed] [Google Scholar]
- Ripamonti C.I., Maniezzo M., Campa T., Fagnoni E., Brunelli C., Saibene G., et al. (2009) Decreased occurrence of osteonecrosis of the jaw after implementation of dental preventive measures in solid tumour patients with bone metastases treated with bisphosphonates. The experience of the National Cancer Institute of Milan. Ann Oncol 20: 137–145 [DOI] [PubMed] [Google Scholar]
- Rodrigues P., Hering F., Campagnari J.C. (2004) Use of bisphosphonates can dramatically improve pain in advanced hormone-refractory prostate cancer patients. Prostate Cancer Prostatic Dis 7: 350–354 [DOI] [PubMed] [Google Scholar]
- Rogers M.J., Gordon S., Benford H.L., Coxon F.P., Luckman S.P., Monkkonen J., et al. (2000) Cellular and molecular mechanisms of action of bisphosphonates. Cancer 88(12 Suppl.): 2961–2978 [DOI] [PubMed] [Google Scholar]
- Rosen L.S., Gordon D., Tchekmedyian N.S., Yanagihara R., Hirsh V., Krzakowski M., et al. (2004) Long-term efficacy and safety of zoledronic acid in the treatment of skeletal metastases in patients with nonsmall cell lung carcinoma and other solid tumors: a randomized, phase III, double-blind, placebo-controlled trial. Cancer 100: 2613–2621 [DOI] [PubMed] [Google Scholar]
- Rosen L.S., Gordon D., Tchekmedyian S., Yanagihara R., Hirsh V., Krzakowski M., et al. (2003) Zoledronic acid versus placebo in the treatment of skeletal metastases in patients with lung cancer and other solid tumors: a phase III, double-blind, randomized trial—the Zoledronic Acid Lung Cancer and Other Solid Tumors Study Group. J Clin Oncol 21: 3150–3157 [DOI] [PubMed] [Google Scholar]
- Rucci N., Teti A. (2010) Osteomimicry: how tumor cells try to deceive the bone. Front Biosci (Schol Ed) 2: 907-915 [DOI] [PubMed] [Google Scholar]
- Ryan C.W., Huo D., Demers L.M., Beer T.M., Lacerna L.V. (2006) Zoledronic acid initiated during the first year of androgen deprivation therapy increases bone mineral density in patients with prostate cancer. J Urol 176: 972–978 [DOI] [PubMed] [Google Scholar]
- Saad F. (2008) New research findings on zoledronic acid: survival, pain, and anti-tumour effects. Cancer Treat Rev 34: 183-192 [DOI] [PubMed] [Google Scholar]
- Saad F., Eastham J. (2010a) Maintaining bone health in prostate cancer throughout the disease continuum. Semin Oncol 37(Suppl. 1): S30–S37 [DOI] [PubMed] [Google Scholar]
- Saad F., Eastham J.A. (2010b) Zoledronic acid use in patients with bone metastases from renal cell carcinoma or bladder cancer. Semin Oncol 37(Suppl. 1): S38–S44 [DOI] [PubMed] [Google Scholar]
- Saad F., Gleason D.M., Murray R., Tchekmedyian S., Venner P., Lacombe L., et al. (2002) A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst 94: 1458–1468 [DOI] [PubMed] [Google Scholar]
- Saad F., Gleason D.M., Murray R., Tchekmedyian S., Venner P., Lacombe L., et al. (2004) Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J Natl Cancer Inst 96: 879–882 [DOI] [PubMed] [Google Scholar]
- Saad F., Lipton A. (2005) Zoledronic acid is effective in preventing and delaying skeletal events in patients with bone metastases secondary to genitourinary cancers. BJU Int 96: 964–969 [DOI] [PubMed] [Google Scholar]
- Saad F., Lipton A., Cook R., Chen Y.M., Smith M., Coleman R. (2007) Pathologic fractures correlate with reduced survival in patients with malignant bone disease. Cancer 110: 1860–1867 [DOI] [PubMed] [Google Scholar]
- Scher H.I., Heller G., Molina A., Kheoh T.S., Attard G., Moreira J., et al. (2011) Evaluation of circulating tumor cell (CTC) enumeration as an efficacy response biomarker of overall survival (OS) in metastatic castration-resistant prostate cancer (mCRPC): planned final analysis of COU-AA-301, a randomized double-blind, placebo-controlled phase III study of abiraterone acetate (AA) plus low-dose prednisone (P) post docetaxel. Oral session presented at: 2011 ASCO Annual Meeting, 4–8 June 2011, Chicago, IL Abstract LBA4517 [Google Scholar]
- Shiozawa Y., Havens A.M., Pienta K.J., Taichman R.S. (2008) The bone marrow niche: habitat to hematopoietic and mesenchymal stem cells, and unwitting host to molecular parasites. Leukemia 22: 941–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small E.J., Smith M.R., Seaman J.J., Petrone S., Kowalski M.O. (2003) Combined analysis of two multicenter, randomized, placebo-controlled studies of pamidronate disodium for the palliation of bone pain in men with metastatic prostate cancer. J Clin Oncol 21: 4277–4284 [DOI] [PubMed] [Google Scholar]
- Smith M.R. (2006) Treatment-related osteoporosis in men with prostate cancer. Clin Cancer Res 12: 6315s–6319s [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M.R., Cook R.J., Coleman R., Brown J., Lipton A., Major P., et al. (2007) Predictors of skeletal complications in men with hormone-refractory metastatic prostate cancer. Urology 70: 315–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M.R., Eastham J., Gleason D.M., Shasha D., Tchekmedyian S., Zinner N. (2003) Randomized controlled trial of zoledronic acid to prevent bone loss in men receiving androgen deprivation therapy for nonmetastatic prostate cancer. J Urol 169: 2008–2012 [DOI] [PubMed] [Google Scholar]
- Smith M.R., Egerdie B., Hernandez Toriz N., Feldman R., Tammela T.L., Saad F., et al. (2009) Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med 361: 745–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M.R., McGovern F.J., Zietman A.L., Fallon M.A., Hayden D.L., Schoenfeld D.A., et al. (2001) Pamidronate to prevent bone loss during androgen-deprivation therapy for prostate cancer. N Engl J Med 345: 948–955 [DOI] [PubMed] [Google Scholar]
- Smith M.R., Saad F., Coleman R., Shore N., Fizazi K., Tombal B., et al. (2012) Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Lancet 379: 39–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltau J., Zirrgiebel U., Esser N., Schachtele C., Totzke F., Unger C., et al. (2008) Antitumoral and antiangiogenic efficacy of bisphosphonates in vitro and in a murine RENCA model. Anticancer Res 28(2A): 933–941 [PubMed] [Google Scholar]
- Taxel P., Dowsett R., Richter L., Fall P., Klepinger A., Albertsen P. (2010) Risedronate prevents early bone loss and increased bone turnover in the first 6 months of luteinizing hormone-releasing hormone-agonist therapy for prostate cancer. BJU Int 106: 1473–1476 [DOI] [PubMed] [Google Scholar]
- Ullen A., Schwarz S., Lennartsson L., Kalkner K.M., Sandstrom P., Costa F., et al. (2009) Zoledronic acid induces caspase-dependent apoptosis in renal cancer cell lines. Scand J Urol Nephrol 43: 98–103 [DOI] [PubMed] [Google Scholar]
- US National Institutes of Health (2009) RADAR trial—randomized androgen deprivation and radiotherapy. Available at: http://clinicaltrials.gov/ct2/show/NCT00193856
- US National Institutes of Health (2011a) Study on prolonging bone metastasis-free survival in men with hormone refractory prostate cancer. Available at: http://clinicaltrials.gov/ct2/show/NCT00286091
- US National Institutes of Health (2011b) Zoledronate plus standard therapy compared with placebo plus standard therapy to prevent bone metastases in patients with recurrent prostate cancer that has no symptoms. Available at: http://clinicaltrials.gov/ct2/show/NCT00005073
- Weckermann D., Polzer B., Ragg T., Blana A., Schlimok G., Arnholdt H., et al. (2009) Perioperative activation of disseminated tumor cells in bone marrow of patients with prostate cancer. J Clin Oncol 27: 1549–1556 [DOI] [PubMed] [Google Scholar]
- Yuasa T., Sato K., Ashihara E., Takeuchi M., Maita S., Tsuchiya N., et al. (2009) Intravesical administration of gammadelta T cells successfully prevents the growth of bladder cancer in the murine model. Cancer Immunol Immunother 58: 493–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaghloul M.S., Boutrus R., El-Hossieny H., Kader Y.A., El-Attar I., Nazmy M. (2010) A prospective, randomized, placebo-controlled trial of zoledronic acid in bony metastatic bladder cancer. Int J Clin Oncol 15: 382–389 [DOI] [PubMed] [Google Scholar]