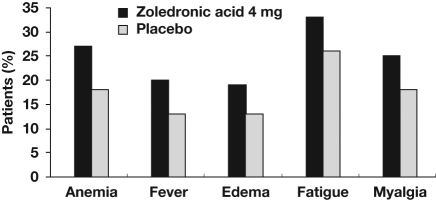
Figure 3.
Most frequently reported adverse events in the phase III placebo-controlled trial of zoledronic acid (4 mg every 3–4 weeks) in men with bone metastases from castration-resistant prostate cancer. Adapted with permission from Parker [2005]. © 2005 John Wiley and Sons.