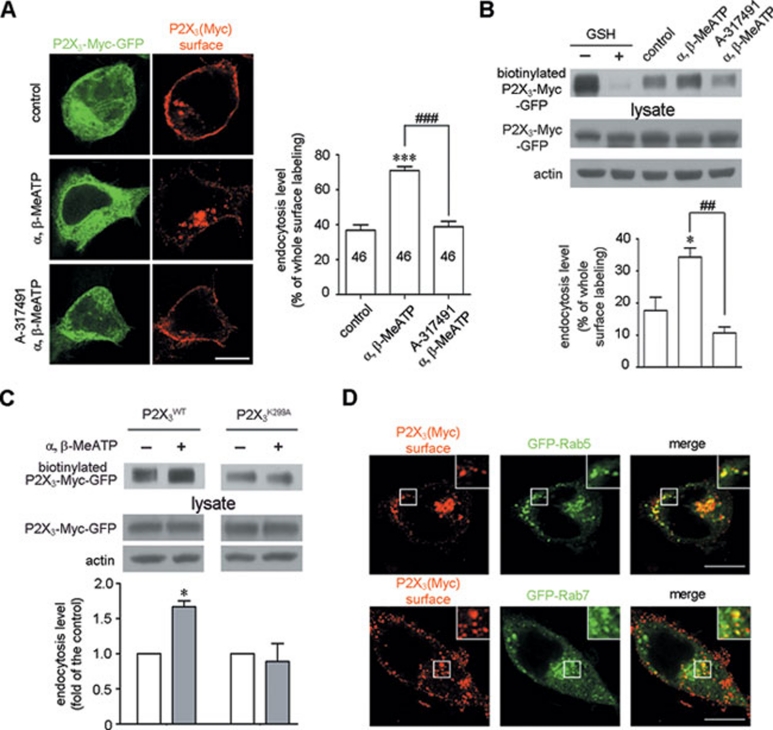
Figure 3.
The endocytosis of P2X3 receptors is ligand dependent. (A) HEK293 cells expressing P2X3-Myc-GFP were surface-labeled with a Myc antibody under non-permeabilized conditions. Representative images showed that α, β-MeATP enhanced the endocytosis of P2X3 receptors, and this effect was abolished by A-317491. Scale bar, 10 μm. The numbers on the bars represent the number of cells per condition. (B) Surface biotinylation was carried out in HEK293 cells expressing P2X3-Myc-GFP following the same treatments as in (A). Lanes 1-2 show that surface-biotinylated P2X3-Myc-GFP was efficiently stripped with glutathione (GSH). Lanes 3-5 show that α, β-MeATP enhanced the endocytosis of P2X3 receptor, which was abolished by A-317491. Actin served as a loading control. (C) α, β-MeATP increased the endocytosis of the wild-type P2X3 receptor, but not the P2X3K299A mutant receptor. (D) Endocytic Myc-labeled P2X3 receptor-positive puncta (red) co-localized with either GFP-Rab5 or GFP-Rab7 (green) in HEK293 cells after α, β-MeATP treatment. The insets are high magnification of the areas with white square frames. Scale bar, 10 μm. *P < 0.05 and ***P < 0.001 versus control, ##P < 0.01 and ###P < 0.001 versus indicated treatment in all experiments; n = 3.