Table 1.
Diels-Alder reactions of diene 1 with dienophiles 4–6 a.
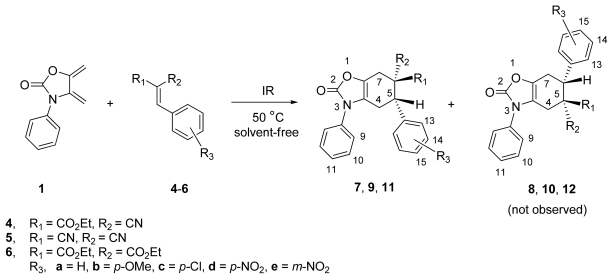 | ||||||
|---|---|---|---|---|---|---|
| Entry | Dienophile | R1 | R2 | R3 | Reaction Time (h) | Product e (%) |
| 1 | 4a | CO2Et | CN | H | 3.5 | 7a (73) |
| 2 b | 4a | CO2Et | CN | H | 20 | 7a (30) |
| 3 c | 4a | CO2Et | CN | H | 24 | 7a (20) |
| 4 d | 4a | CO2Et | CN | H | 24 | 7a (20) |
| 5 | 4b | CO2Et | CN | p-OMe | 4.0 | 7b (50) |
| 6 | 4c | CO2Et | CN | p-Cl | 3.5 | 7c (60) |
| 7 | 4d | CO2Et | CN | p-NO2 | 3.0 | 7d (80) |
| 8 | 4e | CO2Et | CN | m-NO2 | 3.5 | 7e (55) |
| 9 | 5a | CN | CN | H | 4.0 | 9a (80) |
| 10 | 5b | CN | CN | p-OMe | 4.5 | 9b (55) |
| 11 | 5c | CN | CN | p-Cl | 3.0 | 9c (75) |
| 12 | 5d | CN | CN | p-NO2 | 3.0 | 9d (85) |
| 13 | 6a | CO2Et | CO2Et | H | 5.0 | 11a (35) |
| 14 | 6b | CO2Et | CO2Et | p-OMe | 6.0 | 11b (25) |
| 15 | 6c | CO2Et | CO2Et | p-Cl | 5.0 | 11c (30) |
All entries were carried out under IR irradiation at 50 °C and solvent-free conditions, except entries 2–4;
Under thermal (50 °C) and solvent-free conditions;
Under thermal conditions (50 °C) in benzene as the solvent;
Under thermal conditions (50 °C) in THF as the solvent;
After column chromatography.
