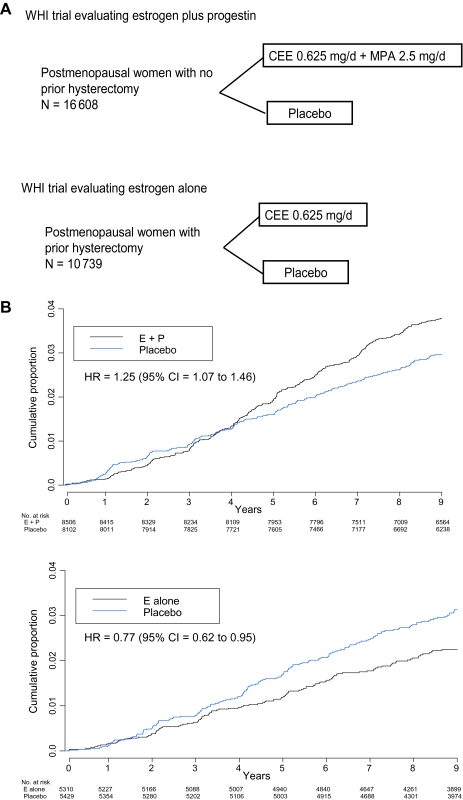
Figure 1.
Women's Health Initiative (WHI) Hormone Therapy Clinical Trials. A) Program design. The depicted study implementation details have been described previously (16–18). Entry criteria for both trials were similar, except that previous hysterectomy was required for estrogen-alone trial entry. Eligible were postmenopausal women aged 50–79 years with anticipated 3 years or greater survival. Exclusions included prior breast or endometrial cancer at any time or other cancer (except nonmelanoma skin cancer) within the previous 10 years. Hormone therapy users at screening were eligible after a 3-month washout. Despite similar eligibility, participant characteristics in the two trials differed, primarily with respect to factors that are associated with hysterectomy. Women in the estrogen-alone trial were heavier and more likely to have had bilateral oophorectomy and to have used hormone therapy in the past (18). Between 1993 and 1998, 10 739 women with prior hysterectomy were randomly assigned to estrogen alone (conjugated equine estrogen [CEE], 0.625 mg/d) or matching placebo and 16 808 women with an intact uterus were randomly assigned to estrogen plus progestin (conjugated equine estrogen, 0.625 mg/d plus medroxyprogesterone acetate [MPA], 2.5 mg/d) or a matching placebo. A mammogram and a clinical breast examination with no evidence of cancer were required for entry. Annual mammography and clinical breast examinations with negative findings were a prerequisite to dispensing ongoing study medication. Breast biopsies were clinically directed. Colorectal cancer screening was not protocol defined, but information on sigmoidoscopy and/or colonoscopy and fecal occult blood testing was collected semiannually. All cancer outcomes were verified by blinded medical record review. Depicted are the study designs of the WHI placebo-controlled clinical trials evaluating estrogen plus progestin and estrogen alone. B) Invasive breast cancer incidence by hormone therapy group. Hazard ratios (HRs) are from Cox proportional hazards regression models, stratified by age and randomization group in the WHI dietary modification trial and prior disease if applicable. CI = confidence interval; E + P = estrogen plus progestin; E alone = estrogen alone.