Abstract
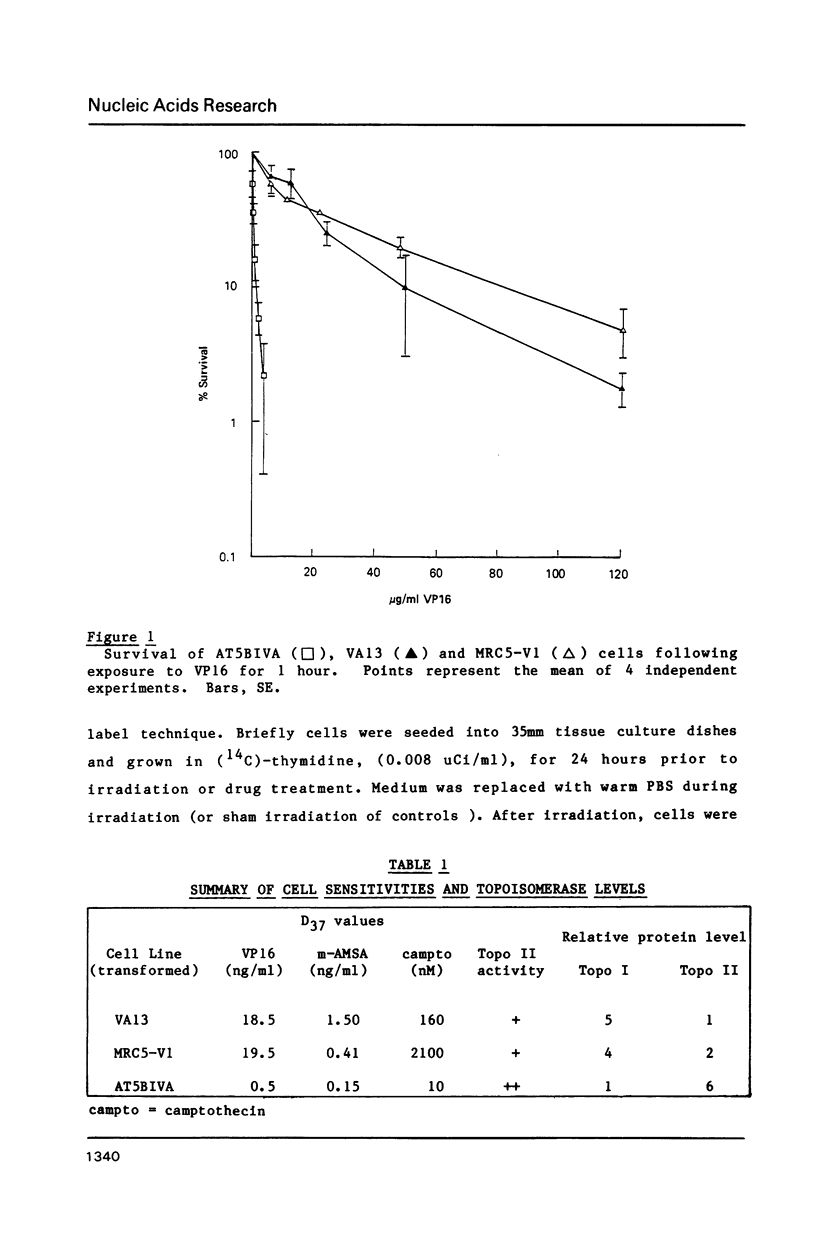
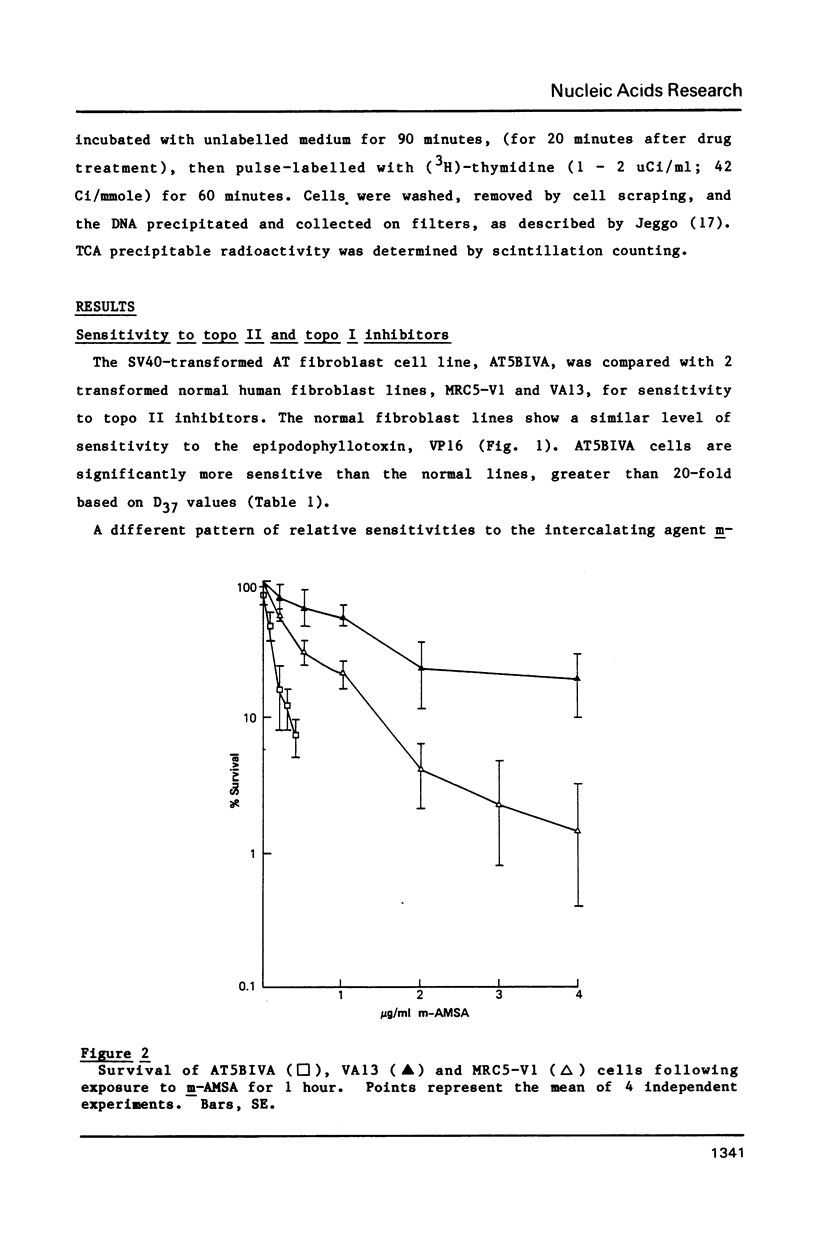
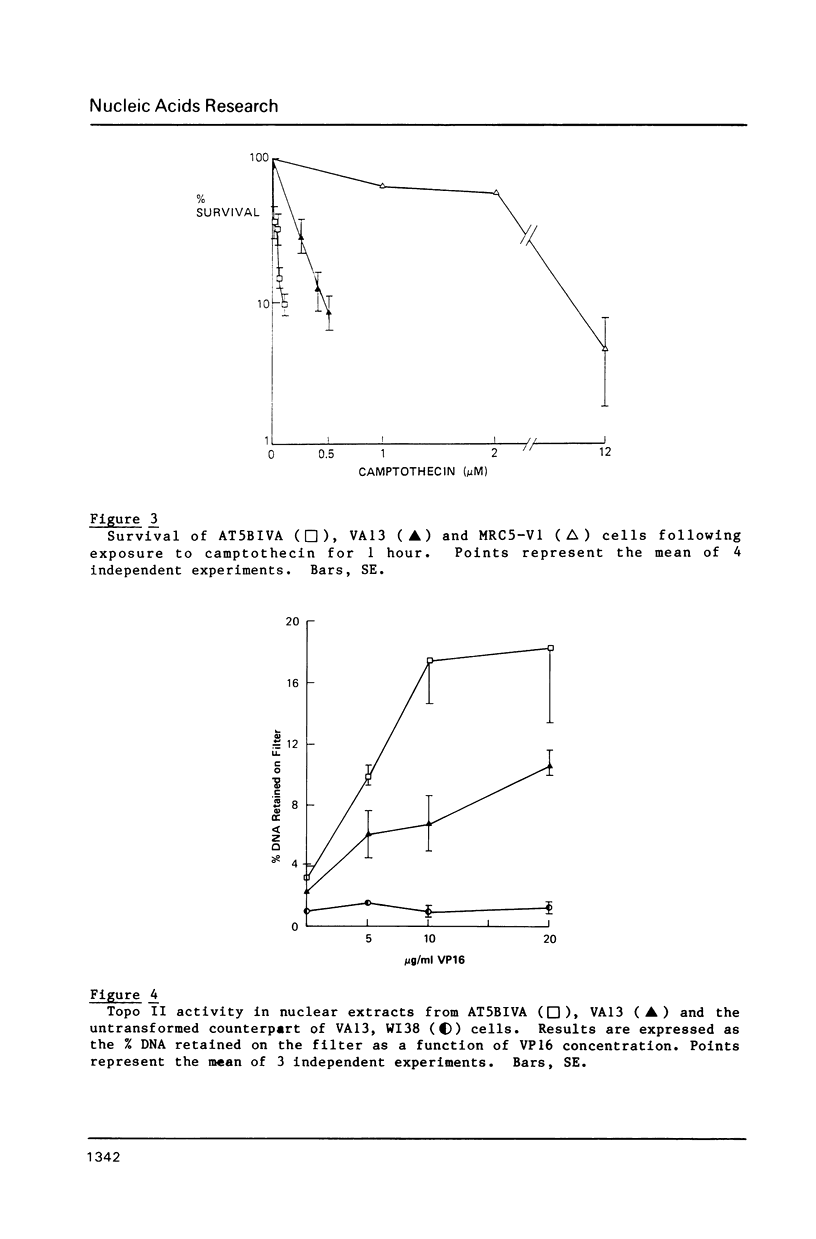
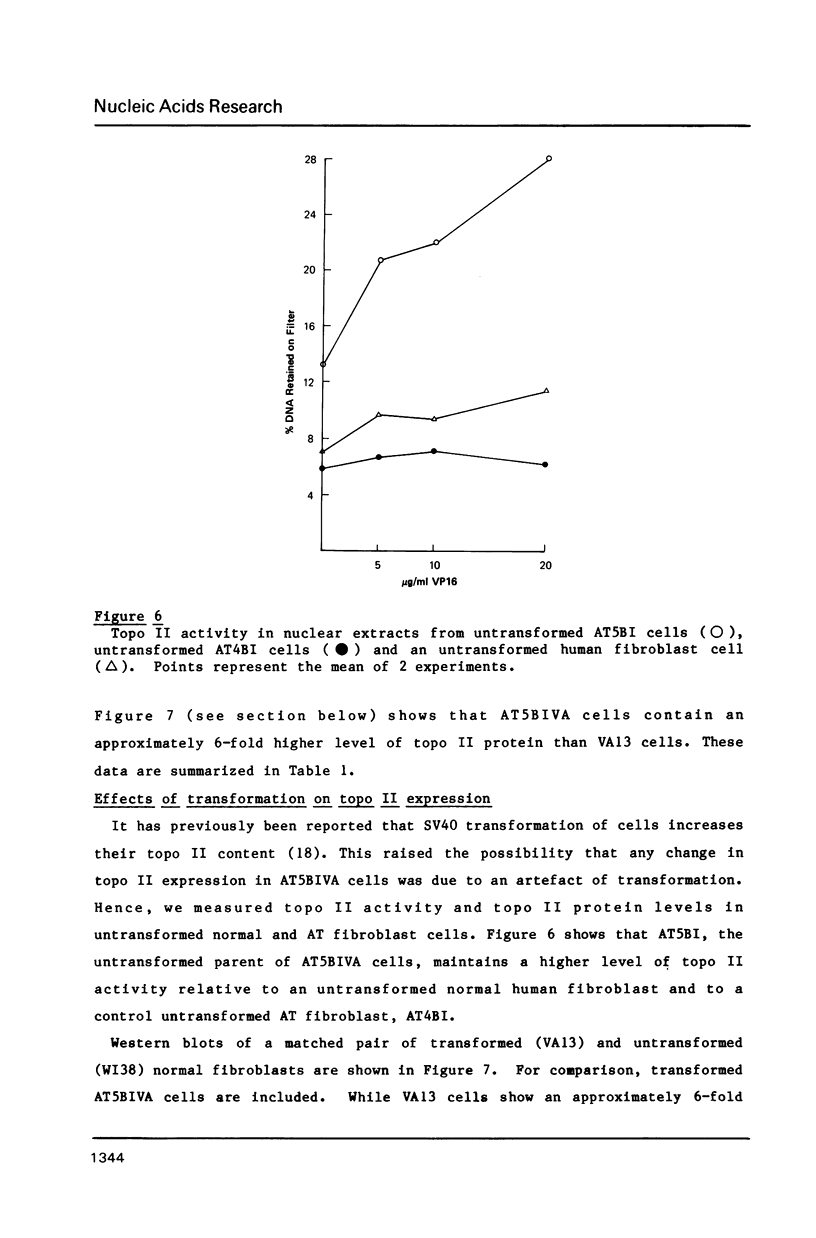
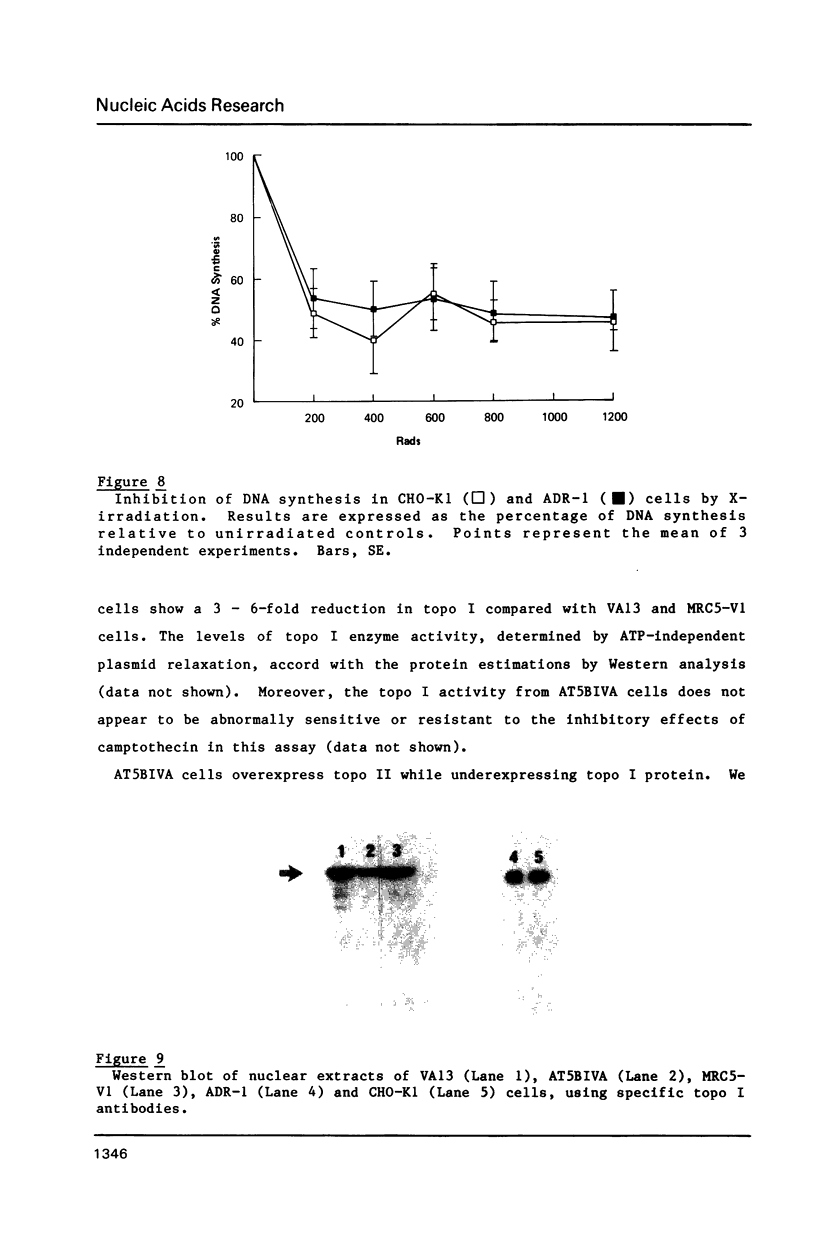
Ataxia telangiectasia (AT) cell lines are characterised by their hypersensitivity to ionizing radiation and bleomycin, and their failure to inhibit DNA synthesis after DNA damage. A recent report [Singh et al. (1988) Nucl. Acids Res. 16, 3919-3929] indicated that a reduction in topoisomerase II (topo II) activity was a feature of AT lymphoblast cell lines. We have studied the possible role of DNA topoisomerases in determining the phenotype of an AT fibroblast cell line. AT5BIVA cells are sensitive to the topo II inhibitors etoposide (VP16) and amsacrine (m-AMSA), compared to normal human fibroblasts (MRC5-V1 and VA13). AT5BIVA cells express a 3-fold higher level of topo II protein than MRC5-V1 cells, and 6-fold higher than VA13. This is reflected in elevated topo II activity in AT5BIVA cells. Untransformed AT5BI cells also show elevated topo II activity compared to untransformed normal cells. The extent of overproduction of topo II in AT5BIVA cells is comparable with that seen in a mutant Chinese hamster cell line, ADR-1, which is similarly hypersensitive to both bleomycin and topo II inhibitors. However, ADR-1 cells show neither hypersensitivity to ionizing radiation nor abnormal inhibition of DNA synthesis following DNA damage. Topo II overproduction per se does not appear sufficient to generate an "AT-like" phenotype. AT5BIVA cells express a reduced level of topoisomerase I (topo I) and are hypersensitive to the topo I inhibitor, camptothecin. ADR-1 cells express a normal level of topo I, indicating that a reduction in the level of topo I is not the inevitable consequence of an elevation in topo II.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chen G. L., Yang L., Rowe T. C., Halligan B. D., Tewey K. M., Liu L. F. Nonintercalative antitumor drugs interfere with the breakage-reunion reaction of mammalian DNA topoisomerase II. J Biol Chem. 1984 Nov 10;259(21):13560–13566. [PubMed] [Google Scholar]
- Cramer P., Painter R. B. Bleomycin-resistant DNA synthesis in ataxia telangiectasia cells. Nature. 1981 Jun 25;291(5817):671–672. doi: 10.1038/291671a0. [DOI] [PubMed] [Google Scholar]
- Crespi M. D., Mladovan A. G., Baldi A. Increment of DNA topoisomerases in chemically and virally transformed cells. Exp Cell Res. 1988 Mar;175(1):206–215. doi: 10.1016/0014-4827(88)90267-4. [DOI] [PubMed] [Google Scholar]
- Davies S. M., Robson C. N., Davies S. L., Hickson I. D. Nuclear topoisomerase II levels correlate with the sensitivity of mammalian cells to intercalating agents and epipodophyllotoxins. J Biol Chem. 1988 Nov 25;263(33):17724–17729. [PubMed] [Google Scholar]
- Glisson B., Gupta R., Hodges P., Ross W. Cross-resistance to intercalating agents in an epipodophyllotoxin-resistant Chinese hamster ovary cell line: evidence for a common intracellular target. Cancer Res. 1986 Apr;46(4 Pt 2):1939–1942. [PubMed] [Google Scholar]
- Gotoff S. P., Amirmokri E., Liebner E. J. Ataxia telangiectasia. Neoplasia, untoward response to x-irradiation, and tuberous sclerosis. Am J Dis Child. 1967 Dec;114(6):617–625. doi: 10.1001/archpedi.1967.02090270073006. [DOI] [PubMed] [Google Scholar]
- Henner W. D., Blazka M. E. Hypersensitivity of cultured ataxia-telangiectasia cells to etoposide. J Natl Cancer Inst. 1986 Jun;76(6):1007–1011. [PubMed] [Google Scholar]
- Hoar D. I., Sargent P. Chemical mutagen hypersensitivity in ataxia telangiectasia. Nature. 1976 Jun 17;261(5561):590–592. doi: 10.1038/261590a0. [DOI] [PubMed] [Google Scholar]
- Houldsworth J., Lavin M. F. Effect of ionizing radiation on DNA synthesis in ataxia telangiectasia cells. Nucleic Acids Res. 1980 Aug 25;8(16):3709–3720. doi: 10.1093/nar/8.16.3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeggo P. A. X-ray sensitive mutants of Chinese hamster ovary cell line: radio-sensitivity of DNA synthesis. Mutat Res. 1985 May;145(3):171–176. doi: 10.1016/0167-8817(85)90024-0. [DOI] [PubMed] [Google Scholar]
- Lehmann A. R., Stevens S. The response of ataxia telangiectasia cells to bleomycin. Nucleic Acids Res. 1979;6(5):1953–1960. doi: 10.1093/nar/6.5.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L. F., Rowe T. C., Yang L., Tewey K. M., Chen G. L. Cleavage of DNA by mammalian DNA topoisomerase II. J Biol Chem. 1983 Dec 25;258(24):15365–15370. [PubMed] [Google Scholar]
- Mattern M. R., Mong S. M., Bartus H. F., Mirabelli C. K., Crooke S. T., Johnson R. K. Relationship between the intracellular effects of camptothecin and the inhibition of DNA topoisomerase I in cultured L1210 cells. Cancer Res. 1987 Apr 1;47(7):1793–1798. [PubMed] [Google Scholar]
- Menzel R., Gellert M. Regulation of the genes for E. coli DNA gyrase: homeostatic control of DNA supercoiling. Cell. 1983 Aug;34(1):105–113. doi: 10.1016/0092-8674(83)90140-x. [DOI] [PubMed] [Google Scholar]
- Minford J., Pommier Y., Filipski J., Kohn K. W., Kerrigan D., Mattern M., Michaels S., Schwartz R., Zwelling L. A. Isolation of intercalator-dependent protein-linked DNA strand cleavage activity from cell nuclei and identification as topoisomerase II. Biochemistry. 1986 Jan 14;25(1):9–16. doi: 10.1021/bi00349a002. [DOI] [PubMed] [Google Scholar]
- Morgan J. L., Holcomb T. M., Morrissey R. W. Radiation reaction in ataxia telangiectasia. Am J Dis Child. 1968 Nov;116(5):557–558. doi: 10.1001/archpedi.1968.02100020561022. [DOI] [PubMed] [Google Scholar]
- Robson C. N., Hoban P. R., Harris A. L., Hickson I. D. Cross-sensitivity to topoisomerase II inhibitors in cytotoxic drug-hypersensitive Chinese hamster ovary cell lines. Cancer Res. 1987 Mar 15;47(6):1560–1565. [PubMed] [Google Scholar]
- Shiloh Y., Tabor E., Becker Y. Abnormal response of ataxia-telangiectasia cells to agents that break the deoxyribose moiety of DNA via a targeted free radical mechanism. Carcinogenesis. 1983 Oct;4(10):1317–1322. doi: 10.1093/carcin/4.10.1317. [DOI] [PubMed] [Google Scholar]
- Singh S. P., Mohamed R., Salmond C., Lavin M. F. Reduced DNA topoisomerase II activity in ataxia-telangiectasia cells. Nucleic Acids Res. 1988 May 11;16(9):3919–3929. doi: 10.1093/nar/16.9.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. J., Anderson C. O., Watson J. V. Predominant role for DNA damage in etoposide-induced cytotoxicity and cell cycle perturbation in human SV40-transformed fibroblasts. Cancer Res. 1986 Nov;46(11):5641–5645. [PubMed] [Google Scholar]
- Sullivan D. M., Glisson B. S., Hodges P. K., Smallwood-Kentro S., Ross W. E. Proliferation dependence of topoisomerase II mediated drug action. Biochemistry. 1986 Apr 22;25(8):2248–2256. doi: 10.1021/bi00356a060. [DOI] [PubMed] [Google Scholar]
- Taylor A. M., Harnden D. G., Arlett C. F., Harcourt S. A., Lehmann A. R., Stevens S., Bridges B. A. Ataxia telangiectasia: a human mutation with abnormal radiation sensitivity. Nature. 1975 Dec 4;258(5534):427–429. doi: 10.1038/258427a0. [DOI] [PubMed] [Google Scholar]
- Tewey K. M., Chen G. L., Nelson E. M., Liu L. F. Intercalative antitumor drugs interfere with the breakage-reunion reaction of mammalian DNA topoisomerase II. J Biol Chem. 1984 Jul 25;259(14):9182–9187. [PubMed] [Google Scholar]
- Wang J. C. DNA topoisomerases. Annu Rev Biochem. 1985;54:665–697. doi: 10.1146/annurev.bi.54.070185.003313. [DOI] [PubMed] [Google Scholar]